
Credit: “Hydrogen bubbles from olivine rocks” prompt, May 21, 2025, via ChatGPT
Background
Synopsis: Hydrogen is the most abundant element in the universe, and it might just be the key to a cleaner energy future. Most hydrogen today is made using fossil fuels, raising a big question: Can we unlock a truly low carbon way to produce it right here on Earth?
- In recent episodes, we learned about the many ways we obtain energy from the Earth and then learned that all these energies are ultimately from the Sun and stellar evolution.
- But there is another form of energy, which is the most abundant element in the universe—hydrogen.
- When hydrogen is burned in air, it releases a lot of energy with the only emissions being water vapor and warm air, making it one of the lowest carbon emissions energy choices.
- Hydrogen is lightweight, energy-dense and clean when used. If hydrogen is to become a tenable energy option, production from non-carbon sources is essential.
- Although hydrogen is the most abundant element in the universe, it is thought to be rare in its pure form on Earth. Instead, it must be produced from hydrogen-containing compounds like water or natural gas.
- To produce hydrogen from water, an electric current is passed through water. With the aid of a catalyst, often platinum, water is separated into hydrogen gas and oxygen gas.
- This process is known as electrolysis and requires electricity to occur.
- Generating hydrogen using electrolysis currently requires more energy input than the usable energy stored in the resulting hydrogen. This energy loss makes the process costly and limits its widespread use in commercial hydrogen production. However, emerging technologies are showing promise in significantly improving electrolysis efficiency, which could make hydrogen production from electrolysis more practical and energy-efficient in the future.
- Fossil fuels comprise chains of carbon and hydrogen known as hydrocarbons. Over 95% of hydrogen production is derived from fossil fuels.
- The most common, efficient and least expensive method to produce hydrogen begins with natural gas which is methane (CH4).
- Natural gas is reacted with high pressure steam, generating a mixture of hydrogen, carbon monoxide and a small amount of carbon dioxide.
- The carbon monoxide is then reacted with water to produce additional hydrogen.
- Natural gas-based hydrogen requires less energy to produce and is less expensive while hydrogen from water is costly, thus industry remains tied to carbon-heavy methods of production.
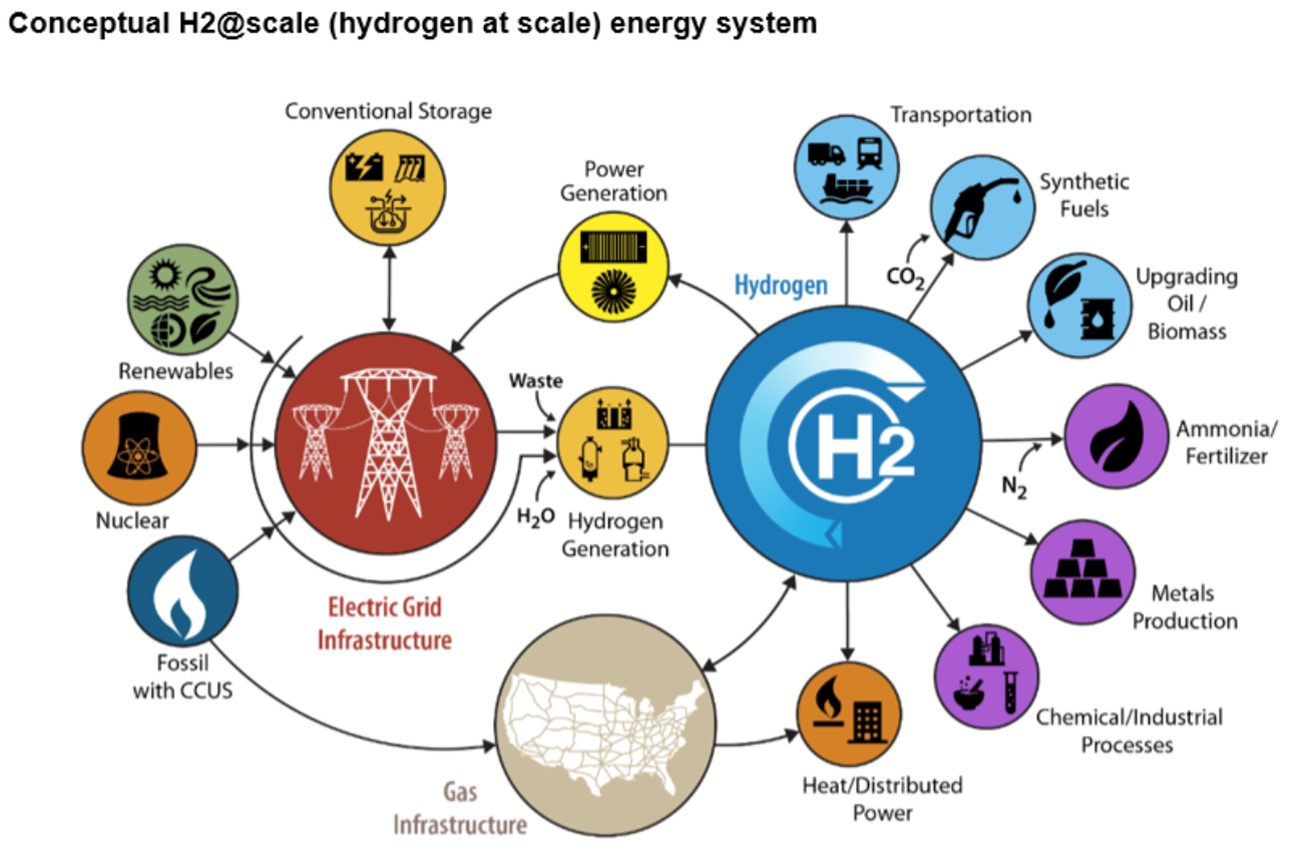
Hydrogen can be produced by various methods with each separating hydrogen from larger molecules. Hydrogen is used in everything from transportation to fertilizers, industry and power.
Credit: U.S. Department of Energy, Hydrogen Program Plan, Figure 3, November 2020
- To produce hydrogen from water, an electric current is passed through water. With the aid of a catalyst, often platinum, water is separated into hydrogen gas and oxygen gas.
- The global demand for hydrogen has been growing steadily over the last twenty-five years.
- Most hydrogen is used by industry to refine petroleum, to produce fertilizer or other chemicals, in metal production and in food production.
- There is a growing interest in using hydrogen fuel cells for electricity production and for transportation alternatives.
- A fuel cell power plant may provide electricity to microgrids in remote locations or individual buildings or facilities.
- As a transportation alternative, the hydrogen fuel cell would be considered a zero-emission vehicle.
- Fuel cells contain more energy per unit weight (greater energy density) than batteries.
- Fuel cells remain expensive, and hydrogen fueling stations are scarce, creating a challenge where limited infrastructure slows demand, and low demand discourages infrastructure investment.
- Although interest in hydrogen is growing, the challenge is scaling up production without increasing greenhouse gas emissions.
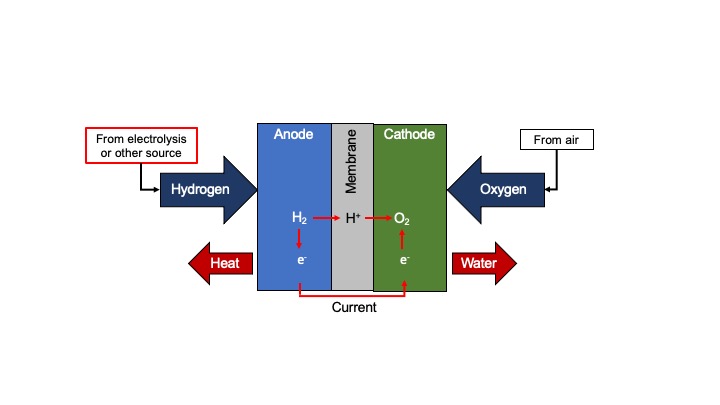
In a hydrogen fuel cell, hydrogen reacts with oxygen producing water and heat.
Credit: Emma Ambrogi, CC BY-SA 4.0, via Wikimedia Commons
- Hydrogen can also form naturally when certain types of rocks—particularly those rich in iron, like olivine—react with water.
- This reaction is known as serpentinization, and it occurs deep within Earth's crust and upper mantle.
- During the process, seawater seeps into rocks such as olivine and pyroxene, causing them to break down into serpentine minerals like antigorite, lizardite, or chrysotile, which often have a fibrous appearance.
- As the minerals transform, the water is oxidized, generating hydrogen gas and releasing heat.
- Serpentinization commonly occurs at mid-oceanic ridges and tectonic plate boundaries and is associated with the formation of hydrothermal vents.

Olivine is found across the globe in various igneous rock and comprises 60% of the upper crust. It has also been found in meteorites, cosmic dust, asteroids, and has been detected on the surface of Mars.
Credit: Hannes Grobe, CC BY-SA 4.0, via Wikimedia Commons - A striking example of naturally occurring hydrogen was discovered in 1987 in Bourakébougou, Mali, where a village well—initially drilled for water—was found to be releasing hydrogen-rich gas.
- Scientists are now exploring the potential of drilling into underground reservoirs to extract this geologic hydrogen, which could offer a clean alternative to fossil fuel-based or electrolysis-based hydrogen production.
- If these underground sources are as widespread as early signs suggest, they may represent a vast, untapped supply of low carbon energy—right beneath our feet.

Fairy circles—circular patches of barren land—are believed to form where hydrogen gas seeps to the surface. Now detectable by satellites, these formations in Namibia may offer new clues in the search for underground hydrogen resources.
Credit: Stephan Getzin, CC BY 2.5, via Wikimedia Commons
- This reaction is known as serpentinization, and it occurs deep within Earth's crust and upper mantle.
- Scientists from the Bureau of Economic Geology at The University of Texas at Austin are exploring a way to produce hydrogen gas directly from iron-rich rocks, providing a carbon-free alternative to hydrogen production.
- The method would rely on using catalysts to speed up the natural process of serpentinization to generate hydrogen gas.
- This approach could open the door to a new sector focused on “geologic hydrogen,” a previously untapped source that might help meet the need for a carbon-free hydrogen source.
- The project is led by researchers Dr. Estibalitz Ukar and Dr. Toti Larson and involves a partnership between UT-Austin and the University of Wyoming.&
- With funding from the U.S. Department of Energy, researchers are expanding their work to test how well this hydrogen-producing method performs in a range of iron-rich rocks found across different parts of North America.
- Specific sites of interest include basalt formations in Iowa, iron-rich deposits in Wyoming and ultramafic rocks in the Midwest—all igneous rocks that contain high amounts of iron and very little silica.
- The grant supports scaling up experiments beyond the lab and exploring the practical potential of the technique in the field.
- To make the reaction more efficient and practical at shallower depths, researchers are experimenting with natural catalysts, like nickel and platinum-group elements, that could speed up hydrogen production.
- If these methods prove successful and scalable, they could help significantly expand global supplies of clean hydrogen, driving the transition away from fossil fuels.
- Hydrogen has the potential to power our future without polluting it—but only if we can find cleaner, more efficient ways to produce it.
- By tapping into Earth's natural processes and accelerating them with scientific innovation, researchers are uncovering promising new paths toward truly carbon-free hydrogen energy.
- The work underway at the Bureau of Economic Geology is one step toward transforming what’s beneath our feet into a cleaner tomorrow.
Episode script
You may have heard that hydrogen could be a fuel of the future, with lower carbon emissions than any we’re using today.
When it’s burned, it produces only water vapor and a lot of useful energy as heat. That sounds pretty good.
And hydrogen is the most abundant element in the universe. That sounds good too.
But nearly all hydrogen on Earth is bound up in other compounds, like water. Or natural gas—methane—which is one carbon atom and four hydrogens.
Today, we get most of our hydrogen by splitting that carbon atom off methane, leaving the hydrogen behind.
But that means making it also makes carbon emissions. This has researchers looking for alternative methods.
The most common is electrolysis. An electric current is run through water, which splits the atoms, releasing oxygen and hydrogen. But it costs a lot of money because it consumes a lot of energy—more than is carried by the hydrogen itself.
There’s another, naturally occurring process that makes hydrogen deep within Earth. When water contacts iron-rich rocks, like olivine and pyroxene, it oxidizes and releases hydrogen gas, and heat.
Scientists at the Bureau of Economic Geology are experimenting with catalysts that could speed up this natural process, but near the surface. This has the potential to release large amounts of hydrogen—without consuming much energy or releasing CO2.
If successful, this process could make hydrogen a fuel of the very near future.

