
What if you’re a fish in freezing water?
Well, a free-swimming ocean fish can migrate to a warmer part of the sea.
But if you’re a lake fish, you don’t have that option. Luckily, the properties of water make its solid form—ice—less dense than its liquid form.
As a lake freezes, the ice floats on the surface, insulating the water below and keeping most lakes—and the fish within them—from freezing solid.
You would still, however, be very cold. Lake fish have to lower their metabolism and enter a state called torpor to reduce their energy demands enough to survive the winter.
This is especially important in spring before the ice melts, when oxygen levels in the water trapped under the ice may be dangerously low.
And what if you’re the unlucky fish where it’s even colder? Fish in polar regions live in saltwater that’s around 28 degrees Fahrenheit—that’s below the freezing point of fish blood.
In that case, you’d need antifreeze in your veins. And polar fish have developed exactly that. They produce a glycoprotein that slows down water’s molecular motion to keep ice crystals from forming.
All pretty interesting if you’re a fish. But what if you’re not?
Medical researchers—and car makers—have teamed up to study fish antifreeze, looking for better ways to keep radiators from icing up and ways to keep human blood and tissue from being damaged by ice crystals when in deep storage. Pretty cool.
Background
Synopsis: How do fish survive in frozen lakes and the waters of Earth’s icy polar regions? Freshwater fish trapped in lakes benefit from the properties of water itself, which cause stratification that insulates the lower layers of water. Their cold-blooded metabolisms slow, but they don’t freeze. Some ocean fish avoid cold temperatures by migrating to warmer waters, but fish that live near the poles have evolved an antifreeze protein that is more effective than car antifreeze, enabling them to live in temperatures below the freezing point of their blood.
- H2O is an amazing molecule that has special properties related to temperature.
- Warm water is less dense, so it floats on cooler water.
- Water is most dense at 39.2°F (4°C), before it freezes solid at 32°F (0°C).
- Solid water (ice) is less dense than liquid water—that’s why ice cubes float.
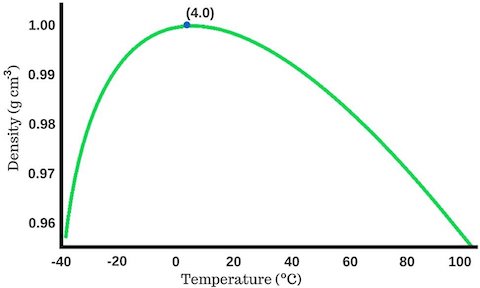
- What happens when lakes freeze?
- Lakes become stratified in the summer with a lower-density sun-warmed layer developing at the top. But as surface waters cool in the fall, they become denser, sinking to the bottom of the lake while pushing the lake’s bottom water to the surface.
- This process, called lake turnover, circulates oxygen and nutrients throughout the water column and is essential for the survival of lake ecosystems.
- When the lake surface finally freezes, layers of ice and snow insulate the underlying layer of denser water well enough to prevent it from freezing solid during the relatively short span of our winters.
- This special property of water preserves a niche for fish to occupy that is warmer than the ice on the surface.
- Below the ice, the body temperature of cold-blooded fish adjusts to the surrounding temperature. In the colder water, their metabolism slows to a point of torpor so they can use as little energy as possible.
- The fish must stay in this state of suspended animation through the winter. The ice layer seals the lake’s water layers, preventing oxygen recharge. By the time the lake thaws in the spring, oxygen levels beneath the ice may be critically low.
- If ice was denser than water, the lake would freeze solid from the bottom up—that would be bad news for the fish.
- But some fish can actually survive being frozen in ice! Fluids in the cells of these fish are salty, causing them to freeze at lower temperatures than ice. In the spring, these fish simply thaw and swim away.
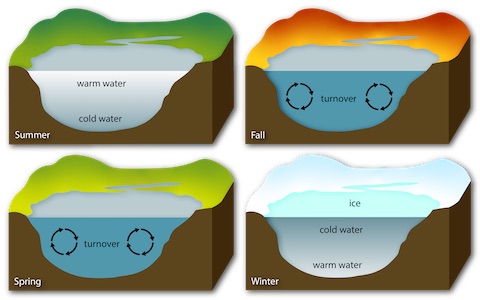
- What happens when oceans freeze?
- Because ocean fish are not trapped by ice, some fish simply migrate to warmer waters for the winter.
- Antarctic seawater is 28.8°F (−1.8°C). Arctic seawater is a consistent 28.6°F (−2.9°C) year-round—almost the freezing point of seawater.
- But fish blood freezes at 30.4°F (−0.9°C), well above polar seawater temperatures. Yet some fish species live year-round in the frigid waters of the Arctic and Antarctic Oceans. Why don’t they freeze?
- Polar fish have evolved a special antifreeze glycoprotein that keeps them from freezing in water that is colder than the freezing point of their blood. Scientists discovered this 50 years ago, but they didn’t know how it worked.
- Recently, chemists used terahertz spectroscopy to examine the behavior of water molecules in the presence of the antifreeze protein. They found the water molecules slowed down dramatically when the protein was around, preventing ice from crystallizing.
- These proteins are more effective than antifreeze. Antifreeze must bond with water molecules to prevent freezing, whereas the proteins just need to be present in a solution to prevent freezing.
- The research was funded by the automobile manufacturer Volkswagen in pursuit of a better alternative to the antifreeze additive used in automotive engines.
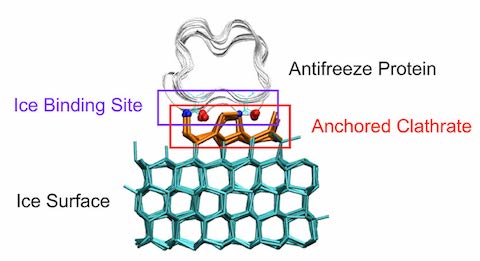
- Scientists are working on ways to use glycoproteins for more than just automotive antifreeze.
- Donated blood and human organs have limited shelf lives, so cryopreservation can be used to extend their viability. But freezing and thawing can damage cells, so organic solvents must be used to prevent ice from forming. Unfortunately, these solvents are toxic and may cause dangerous side effects.
- Scientists are studying how they might use nature’s solution of polar fish glycoproteins to store human tissue in sub-zero environments. Someday this technology could save your life or the life of someone you love.

