
Credit: NASA, ESA, CSA, STScI, and the Webb ERO Production Team
Nearly everything in the universe was made by stars.
Stars are fueled by nuclear fusion. They fuse atoms of the lightest element, with only one proton—hydrogen—into the next lightest element, with two protons—helium.
In every fusion reaction, they’re creating an atom of a new element.
When they’ve used up their hydrogen, they begin to fuse the remaining helium into a heavier element, carbon. Then they’ll fuse that into oxygen and progressively heavier elements.
Each of these fusion reactions is less energetic, so by the time the star is fusing iron, it’s nearing the end of its life.
The star may soon explode in a supernova, dispersing the elements it created into other solar systems where they may be used to form planets.
After a supernova, the star may continue to exist as a black hole or an extremely dense neutron star. In this environment, elements like iron can capture free neutrons to form still heavier elements like silver, gold or plutonium.
The atoms that came together to form Earth 4.5 billion years ago were once part of stars that lived and died long before.
Almost every atom in your body is billions of years old, from the calcium in your bones to the carbon in your DNA, recycled countless times.
That song was right: we are stardust. Stars made the elements that make us.
Background
Synopsis: While ancestry and family trees are interesting, our ultimate origin story is in the stars. All the atoms in the universe formed billions of years ago during the Big Bang and in subsequent supernovas. Those atoms were recycled through multiple galactic events until they formed Earth 4.5 billion years ago. Once life emerged on Earth, the atoms were recycled millions of times to form you. Songwriter Joni Mitchell was right, we are stardust.
- After the Big Bang, 13.787 billion years ago, the early universe consisted of the lightest two elements, hydrogen (atomic number 1), and helium (2). Lithium (3) followed.
- Carbon (6) and oxygen (8) joined the fray between 12 and 7 billion years ago.
- The formation of the additional elements that further enriched the universe occurred through different processes in the life cycles of multitudes of stars.
- A highly simplified list of these processes follows:
- The primary mechanism for the creation of heavier elements is stellar nucleosynthesis, which takes place within the cores of stars.
- As stars age, they undergo nuclear fusion, where two or more atomic nuclei are smashed together to build a heavier nucleus. When hydrogen fuses into helium in a star, it releases tremendous amounts of energy that power the star.
- As a star continues to evolve, it can undergo further fusion reactions, progressively synthesizing heavier elements but producing less and less energy with each step. For example, helium nuclei can fuse to form carbon, and carbon can fuse to form oxygen and other elements.
- The most massive stars can go even further, synthesizing elements up to iron through successive exothermic fusion reactions as we described in ED-091 Our Most Common Element.
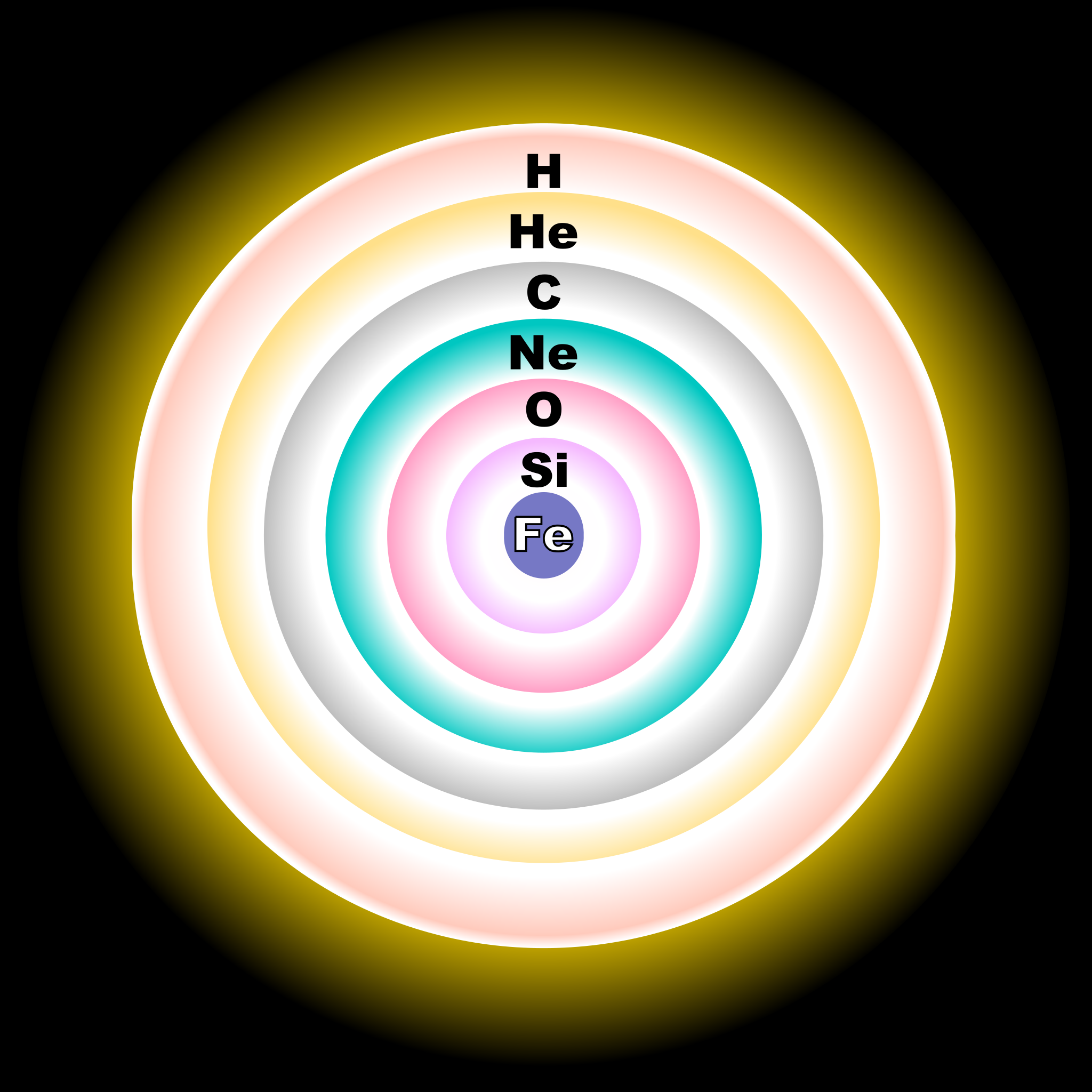
This diagram shows a simplified (not to scale) cross section of a massive, evolved star (with a mass greater than eight times that of the Sun). Where the pressure and temperature permit, concentric shells of hydrogen (H), helium (He), carbon (C), neon/magnesium (Ne), oxygen (O) and silicon (Si) plasma are fusing inside the star. The resulting fusion by-products rain down upon the next lower layer, building up the shell below. As a result of silicon fusion, an inert core of iron (Fe) plasma can steadily build up at the center. Once this core reaches a certain mass, the iron can no longer sustain its own mass.
Credit: Rursus, CC BY-SA 3.0, via Wikimedia Commons - However, for elements heavier than iron (26), energy must be added because those reactions are endothermic.
- Once a star begins fusing to iron, it has exhausted most of its limited nuclear fuel supply and is nearing the end of its life.
- Some stars simply cast their outer layers off into the cosmos and become a small, hot core called a white dwarf, but when more-massive stars reach the ends of their lives, they typically explode in cataclysmic events known as supernovas.
- During a supernova explosion, a star releases an enormous amount of energy, along with a blast wave that ejects the star’s outer layers into space, including its oxygen (8), magnesium (12) and potassium (19).
- Sometimes white dwarves can interact with other stars in binary systems to cause different types of supernovas that produce elements like calcium (20), zinc (30) and manganese (25).
- Supernovas disperse the elements synthesized by the star throughout the galaxy, enriching the surrounding interstellar medium.
- This ejected material can later become part of new star-forming regions and contribute to the formation of subsequent generations of stars and planetary systems.
- Heavier elements can also be produced through a process called neutron capture in even more powerful explosions.
- This occurs when atomic nuclei, such as those of lighter elements like iron, capture free neutrons in an environment with high neutron density. Neutrons can enter a nucleus more easily than charged particles.
- Supernovas leave behind either a black hole or a neutron star, the superdense core of an exploded star. The collision of neutron stars can produce elements like silver (47), iodine (53), platinum (78), gold (79), uranium (92) and plutonium (94) by neutron capture.
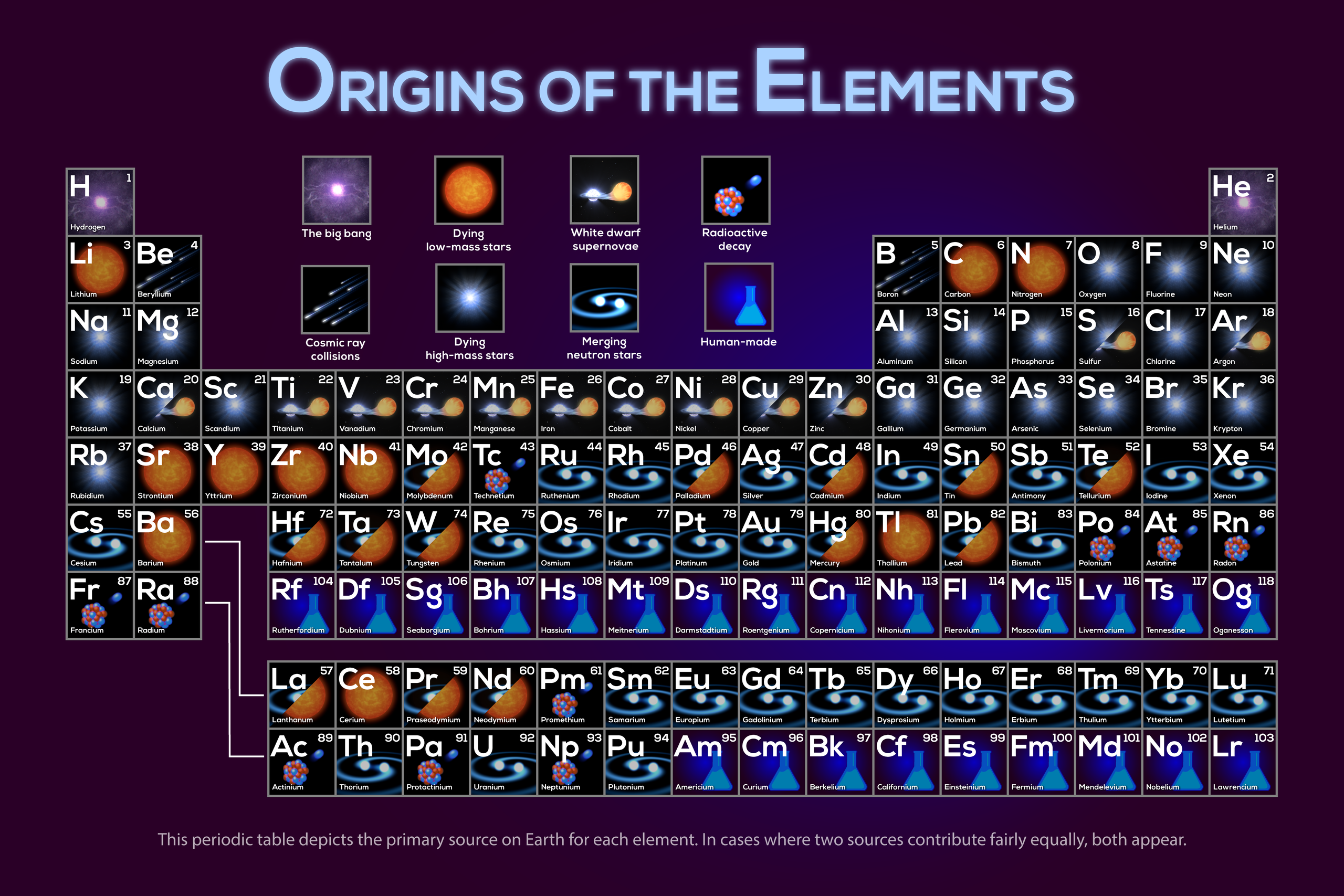
Periodic table showing the most likely origins of each element, ranging from the Big Bang to star-related processes including dying low-mass stars, dying high-mass stars, white dwarf supernovas, and merging neutron stars. Cosmic ray collisions, radioactive decay, and human-made elements round out the origins of other elements. Note that this graphic is a first-order simplification of an active research field with many open questions.
Credit: NASA
- There are a few rarer ways that elements are formed:
- Cosmic rays can collide with atoms, breaking them apart to produce elements like beryllium (4) and boron (5) that were not formed during stellar nucleosynthesis.
- Radioactive elements decay to produce simpler daughter products.
- Elements heavier than plutonium (Pu, atomic number 94) are typically short-lived and must be created through fusion experiments by humans in labs.
- The atoms that came together to form Earth 4.54 billion years ago were once part of stars that lived and died before Earth formed, possibly enduring several supernovas along the way.
- The constant processing of elements in the universe is known as galactic chemical evolution. Every element can be combined in different ways to form compounds, minerals, asteroids, planets, water and, ultimately, life.
- Almost every atom in your body is billions of years old, from the calcium in your bones to the carbon in your DNA, recycled countless times.
This chart accounts for 99.9% of the elements in the typical human body. Except for hydrogen that originated during the Big Bang, 13.7 billion years ago, all other elements were made during the lives and death throes of stars.
Credit: Natural History Museum - As Carl Sagan once said, “The cosmos is within us.”
- And as Joni Mitchell famously wrote (although she was several billion years off):
“We are stardust
Billion-year-old carbon
We are golden
Caught in the devil’s bargain
And we've got to get ourselves back to the garden”
Credit: Original final chorus lyrics © 1969 Crazy Crow Music (Renewed)
Prior to its release on any album, Mitchell performed her song “Woodstock” at the 1969 Big Sur Folk Festival, one month after Woodstock. This recording was made live in studio by the BBC in London on October 9, 1970. Four artists or bands recorded the song in 1970, including, most famously, Crosby, Stills, Nash, and Young.

