If you’re driving while listening to this, please think of your vehicle not as a mere car or SUV, but as a starship cruiser!
Here’s why: the iron it’s made of came from the heart of a distant star.
Stars begin their lives as giant balls of gas, mostly hydrogen, the first element on the periodic table, with one proton.
Under the force of the proto-star’s enormous gravity, hydrogen atoms fuse together to produce helium, with two protons.
This nuclear fusion releases a huge surge of energy, and the star is born.
Hydrogen continues to fuse into helium, releasing more and more energy.
Helium atoms then fuse into carbon atoms, which fuse into silicon atoms, with each subsequent element being heavier.
All this nuclear fusion releases more energy than it takes to fuse the atoms together. And so, the process continues, for millions of years…until the elements fuse into iron.
At that point, it would take more energy to fuse iron into something else than the resulting reaction would produce.
So fusion stops, and the star begins to die.
Soon, the gravity of its iron core becomes so strong that the star collapses on itself, then explodes outward in a supernova, scattering iron across the universe…which eventually forms planets like ours. And our cars.
Background
Synopsis: Why is iron, which is so important to modern civilization, the most abundant metal on Earth? Because it is the heaviest element produced during fusion without having to add energy, and it is the lightest element produced during fission without having to add energy. Energy-wise, everything in the universe wants to be iron!
- Iron is the most abundant element on Earth, making up 34.5 percent of Earth’s mass.
- Oxygen (30 percent), silicon (15 percent), and magnesium (13 percent) follow iron; together, these four elements make up more than 90 percent of Earth’s mass.
- The reason why iron is so plentiful goes right back to the formation of the first stars and iron’s position at the tipping point between energy producing fusion and fission reactions.
- Nuclear fusion occurs when the nuclei of lighter elements are crushed together to form heavier elements.
- For lighter elements, fusion reactions produce energy, like the energy that powers the sun. These are called exothermic reactions.
- For elements heavier than iron, energy must be added or the fusion reaction will not occur. These are called endothermic reactions.
- Nuclear fission operates the opposite way, occurring when heavier nuclei are split into smaller nuclei.
- Unlike fusion reactions, for lighter elements the fission reaction is endothermic—it won’t occur without adding energy.
- For elements heavier than iron, the fission reaction is exothermic—it produces energy.
- Right after the Big Bang, matter was packed together densely, and temperatures were in the millions of degrees.
- During this phase, fusion produced the first five elements on the periodic table: hydrogen, helium, lithium, beryllium, and boron.
- After that, the elements from carbon (6) to iron (26) were created in the cores of stars by exothermic fusion reactions driven by the crush of their own gravity.
- Stars start out as balls of hydrogen that produce and are supported by the energy from fusion.
- First, hydrogen (atomic number 1) fuses to form helium (atomic number 2) and energy. When the hydrogen runs out, the helium fuses to create carbon (6) and energy, and so on, skipping up the periodic table.
- But there is a catch: each reaction requires more energy to start fusion, and each reaction produces less energy from the fusion.
- Once the 28 protons and neutrons of silicon fuse to make a nucleus with an atomic weight of 56 (iron plus energy), that’s the critical tipping point—the energy needed and the energy produced just about offset each other. Any fusion with elements beyond that point simply consumes the star’s energy.
- When smaller stars like our sun exhaust their nuclear fuel, they expand to become red giants, then shed their outer layers and contract to become dense white dwarves— sometimes called zombie stars because they can increase their mass by stripping material from a nearby star, turning their victims into the bare cores of white dwarfs like themselves.
- Sometimes white dwarfs collide with each other, combining their masses. If a white dwarf gains too much mass, it may become unstable, exploding as a type 1a supernova. This thermonuclear explosion totally disrupts the white dwarf, converting most of its mass into iron—probably the principal source of iron in the universe.
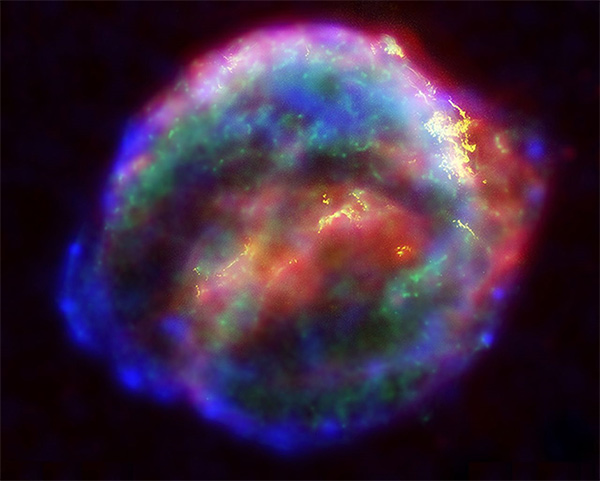
- The famous Kepler Supernova was a type 1a supernova. Discovered by Johannes Kepler in 1604, people at the time could observe it without a telescope, which had not yet been invented. Today, astronomers utilize NASA's three Great Observatories to analyze the supernova remnant in infrared, optical, and X-ray light.
- Without an energy source, more massive stars eventually die after the fusion of silicon to iron. The iron core collapses to make a neutron star, and the collapse energy explodes the outer portions of the star as a supernova.
- Nuclear fusion occurs when the nuclei of lighter elements are crushed together to form heavier elements.
- Iron produced by the death of stars is a key component in the formation of planets. We’ve mentioned its abundance on Earth, but iron is also so plentiful on Mars that the planet shines back at us in beautiful shades of bright red!
- Elements heavier than iron form by a variety of other means, including neutron capture, perhaps created by colliding neutron stars.

