
Credit: Christopher from Salem, OR, USA, via Wikimedia Commons
Something’s happening high in the mountains, invisible to humans—that can dramatically affect our water supply.
It’s a process called sublimation, where a substance goes from its solid form directly to a gas, skipping the liquid state altogether. And water is one of the few substances on Earth that can do this.
It takes 80 calories of energy to melt a gram of ice into liquid water. Then another 540 calories to boil it into gas. But when the environment provides those 620 combined calories all at once, ice can go straight to vapor.
And high mountains are the perfect environment for this to occur, where glaciers and snowpack, exposed to bright sunlight and blown over by warm, dry winds, will often vaporize directly into the atmosphere.
The ice is literally blowing away.
This is important because glaciers and snowpack are nature’s water towers. We depend on their meltwater to fill rivers and our reservoirs, where we use it for municipal water, agriculture and industry.
To calculate the water available to us, scientists analyze the volume stored in the mountains. But sublimation is very difficult to predict, making our water supply hard to measure.
It’s a seemingly simple problem that, especially in places like the American West, impacts millions of lives.
Yet another example of where better data and advancing technology could have a very practical application.
Background
Synopsis: Sublimation is vaporization of a solid without passing through a liquid stage, requiring special conditions of pressure and temperature. Only a few materials change directly from a solid to a gas on Earth’s surface, including dry ice (carbon dioxide, CO2), moth balls, and ice (solid water). Freeze-dried foods are produced by sublimation of their water. Sublimation of snow is challenging for scientists to quantify as they calculate water budgets for key rivers—a big concern for all of us.
- We are accustomed to thinking about materials melting (solid to liquid phase change) and evaporating (liquid to gas phase change), or gases condensing to form liquids that may freeze. But there are transitions that skip the liquid phase altogether that are less well known: sublimation of solids and the opposite, deposition of gases.
- You may have seen sublimation in action if you have witnessed the CO2 fog produced from dry ice, which is solid CO2.
- If you have ever smelled moth balls (solid naphthalene), you are experiencing the sublime scent of naphthalene gas that has evolved from the solid at room temperature.
- You may have also noticed ice cubes shrinking in your freezer, and you may have tried freeze-dried food, both the result of sublimation of solid ice into water vapor.
- And you may have seen snowflakes or winter hoarfrost on windows, both the result of direct deposition of water vapor into solid ice.

Snowflakes form as crystalline solids directly from gaseous water vapor in the air. (ED-194 How Snowflakes Grow)
Credit: Alexey Kljatov, via Wikimedia Commons
- When a material changes from a solid, liquid or gas to a different phase without undergoing a chemical reaction, scientists say a phase change has occurred.
- Melting and freezing, evaporation and condensation, and sublimation and deposition are all examples of phase changes.
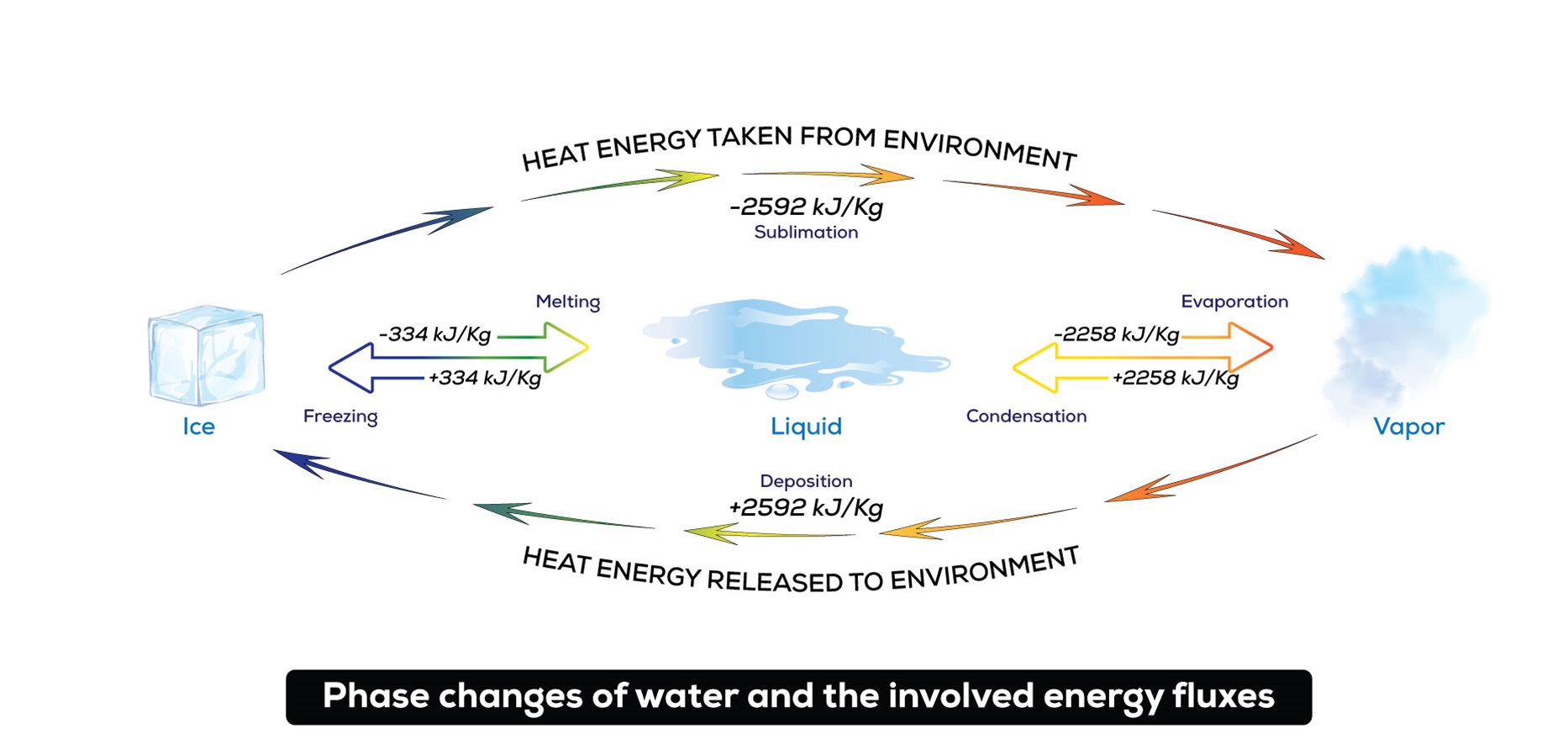
Phase changes possible for water (H2O). To change from solids to gases, energy must be added to the system, and to change from gases to solids, energy is released into the environment. Note that the heat energy required for sublimation (620 calories) is exactly the same as the sum of the heat energy required for melting (80 calories) plus evaporation (540 calories).
Credit: Kh1604, CC BY-SA 4.0, via Wikimedia Commons - Molecular substances transition among phases given the right pressure-temperature combinations.
- Melting and freezing, evaporation and condensation, and sublimation and deposition are all examples of phase changes.
- Phase diagrams describe the conditions required for phase changes to occur in molecular substances.
- These diagrams show the phase that is stable at particular pressures and temperatures.
- The lines between the colored regions describe the precise conditions when two phases can exist in thermodynamic equilibrium.
- The triple point occurs at the temperature and pressure combination necessary for all three phases to exist in thermodynamic equilibrium.
- The critical point occurs where the liquid and gaseous phases of a substance merge together into a single phase. Beyond the temperature of the critical point, the merged single phase is known as a supercritical fluid.
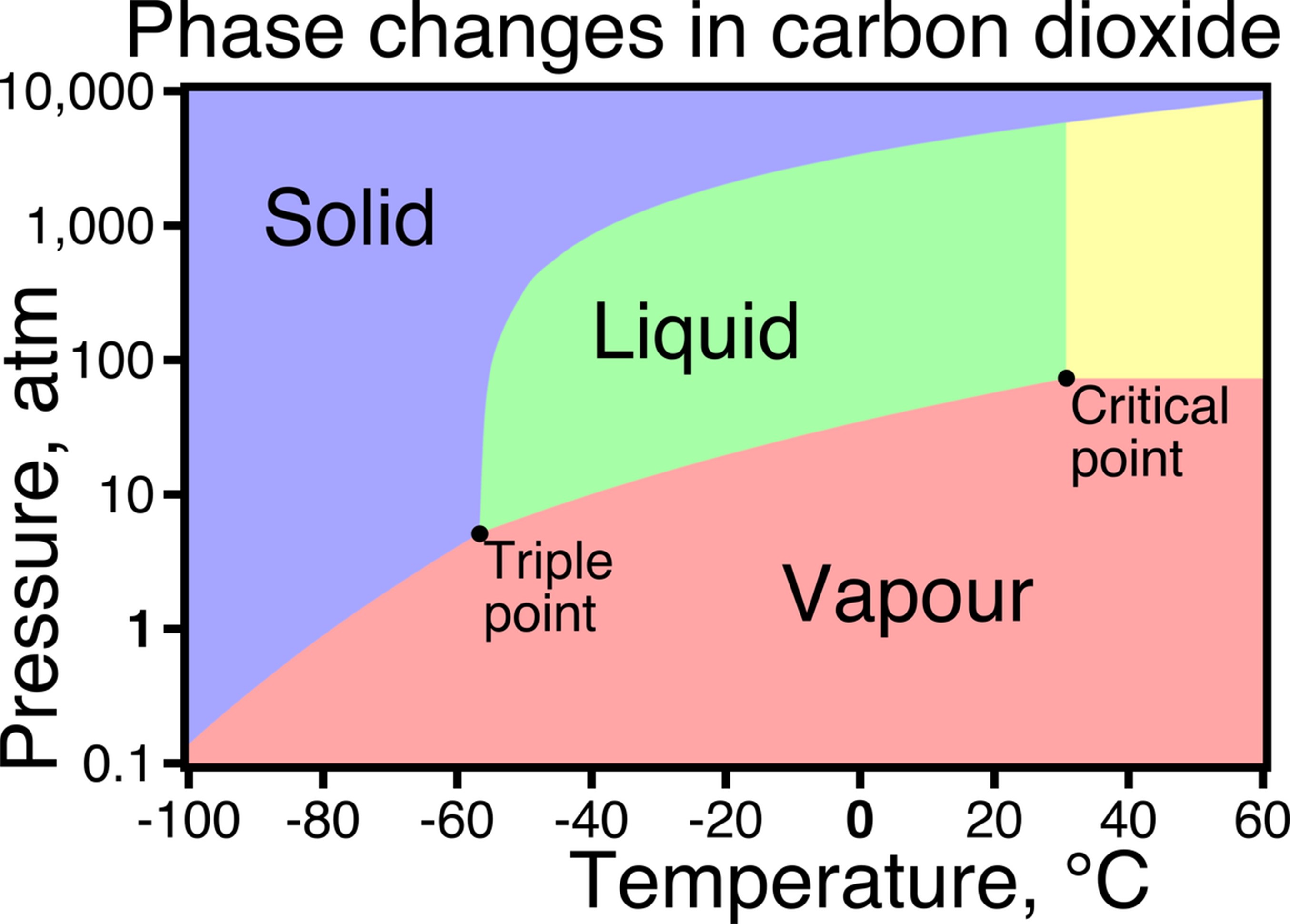
Phase diagram of carbon dioxide (CO2), with pressure on the vertical log scale and temperature on the horizontal linear scale. At one atmosphere of pressure (sea level) and room temperature, dry ice sublimates directly into CO2 fog. Liquid CO2 can’t exist at pressures below 6.1 times that of sea level.
Credit: Foobaz, public domain, via Wikimedia Commons - Most materials exist as a single phase in the pressure-temperature range common on Earth’s surface. For example, salt is a solid and mercury is a liquid at room temperature, but they can exist as other phases at different pressure-temperature combinations.
- Scientists use sublimation to purify certain compounds, similar to distillation.

Ferrocene crystals deposited on a rod after purification by sublimation.
Credit: Bucki at German Wikipedia, public domain, via Wikimedia Commons
- Sublimation occurs as an endothermic reaction when temperatures and pressures are below the triple point for a substance.
- For sublimation to occur, heat must be taken from the environment to provide enough energy for some molecules to escape from the solid into the vapor phase by overcoming the molecular attraction of nearby molecules.
- Sublimation occurs when the atmospheric pressure is too low for a material to exist as a liquid. Otherwise, its mechanism is identical to evaporation.
- Water is a special substance (ED-141 Why Is Ice Slippery?) with a special phase diagram because of the unique behavior of H2O as it turns from a disorganized liquid into a highly organized solid.
- At sea level (1 atmosphere of pressure), H2O can sublimate from ice to water vapor at temperatures below 32°F (0°C). At elevation, pressures drop below 1 atmosphere, so sublimation is even more common in the mountains.
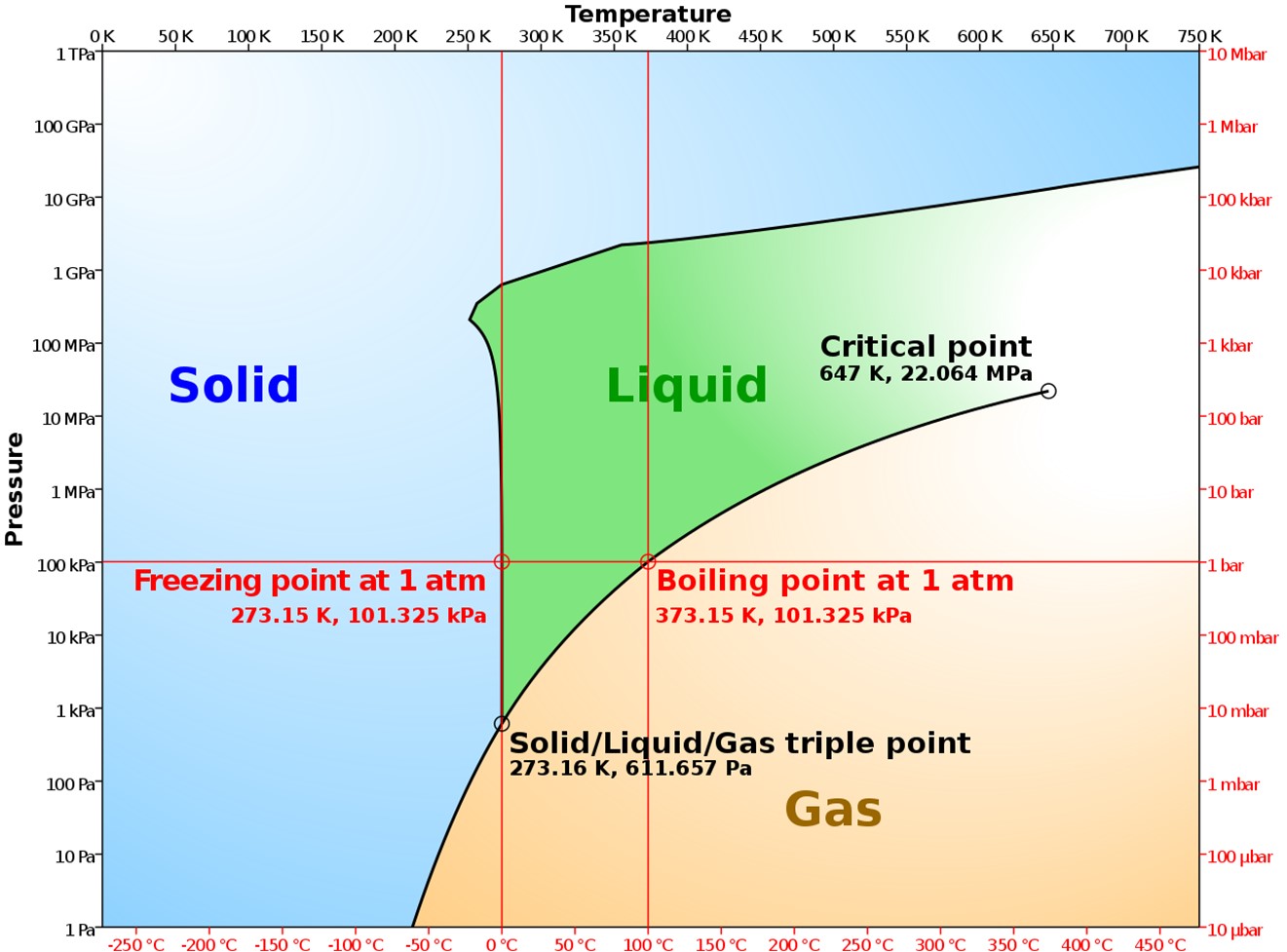
Phase diagram of water as a log-linear chart. Sublimation occurs at pressures and temperatures below the triple point of water.
Credit: Cmglee, via Wikimedia Commons - Sublimation of H2O is apparent in your very own freezer when old ice cubes shrink and when food dehydrates, developing freezer burn.
- Freeze-dried foods intentionally preserve consumables through the dehydration of frozen food by sublimation of its water at reduced pressure or vacuum.
- At sea level (1 atmosphere of pressure), H2O can sublimate from ice to water vapor at temperatures below 32°F (0°C). At elevation, pressures drop below 1 atmosphere, so sublimation is even more common in the mountains.
- Sublimation is intimately linked to Earth’s water cycle, especially where snow and ice are key sources of water for civilization.
- Snowpack and glaciers are nature’s versions of water towers, storing H2O as a solid (ED-206 The Value of Snowpack).
- Water managers use snow water equivalent (SWE) maps to estimate how much snow will melt into its liquid water form to fill the rivers and reservoirs that irrigate our crops, produce our electricity and slake our thirst.
- Colorado River snowpack in 2021 was estimated to be about 80% of average, but streamflows were only 30% of average. Where did all that water go?
- There are a lot of variables to consider in the calculation.
- Evaporation to the atmosphere is a concern but estimates of evaporation are made based on relative humidity during the snowmelt and summer seasons.
- Soil uptake depends on soil dryness as it soaks in the snowmelt. Are drought-parched soils sucking up the water?
- Sublimation creates the greatest uncertainty, because it operates throughout the winter season and is enhanced at the low pressures of higher mountain elevations. Did the snow vaporize directly into the sky?
- Snowpack and ice sublimation get going on low-relative-humidity, sunny days that provide the heat energy to drive the phase change. The process is accelerated by strong dry winds that erode and expose more snow and glacial surface area to the elements.
- The resulting water vapor turns into precipitation that rejoins the system, further complicating modeling efforts.

Sun and wind contribute to sublimation of snow in the Slate River Valley of Colorado. In a nearby valley, scientists are collecting a large variety of data to aid our understanding of the sublimation of snow.
Credit: Juli Hennings, UT Bureau of Economic Geology - From 2022 to 2023, researchers collected volumes of data in valleys near the headwaters of the Colorado River in hopes of better understanding snow sublimation. Analysis is underway.
- The resulting water vapor turns into precipitation that rejoins the system, further complicating modeling efforts.

