
Once an ice skater gets going, friction between her skate and the ice creates a microscopic layer of water that allows the skate to hydroplane.
But before she can get up to speed, and friction can melt the ice, it’s still slippery enough for her to start her glide. Why is ice so slippery?
In the 1800s, scientist Michael Faraday conducted experiments to show that ice, even well below freezing, has a very thin layer of water on its surface.
But the technology to see this layer did not exist. Nor did the scientific understanding to prove that it was there.
It would be more than 100 years before scientists could finally see Faraday’s water layer using X-ray imaging. And still later that they could measure it.
Turns out this thin layer is very thin indeed—thousands of times thinner than a sheet of paper. In fact, it’s just a couple of molecules thick.
When water freezes, its molecules interlock tightly to create the crystalline structure of ice, held together by four hydrogen bonds.
But the molecules on the surface of ice can only bond to the molecules just beneath them, with just three hydrogen bonds. This won’t allow a stable crystalline surface.
This strange, disordered molecular state of water on the surface of ice will persist down to -36o Fahrenheit.
But if the temperature goes below that, ice will no longer be slippery—sometimes with disastrous results, which we’ll talk about on another EarthDate.
Background
Synopsis: Ice is the solid phase of water. Anyone who has experienced a cold winter knows that ice is quite slippery, allowing things like the blade of a skater’s skate to glide instantaneously and effortlessly across solid ice. Scientists have known for more than 150 years that ice’s extremely low coefficient of friction is caused by an ultrathin coating of liquid water on its surface that enables us to skate, ski and slide over a wide range of winter temperatures. But how does this layer form and how can liquid water possibly exist below freezing temperatures?
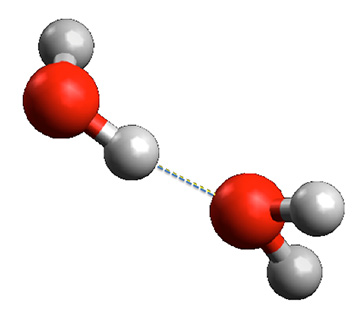
- The amazing H2O molecule has special properties.
- It looks like a Mickey Mouse head, with the single doubly-negatively-charged oxygen atom as the face and the two positively-charged hydrogen atoms as the ears.
- This structure makes it into a tiny electric dipole, with a negative pole on the oxygen end and a positive pole on the hydrogen end. This polar structure is the origin of the binding force between water molecules, which is called hydrogen bonding.
- Water molecules are strongly attracted to each other and clump tightly together in liquids because of hydrogen bonding. This disorganized clustering of liquid water molecules creates the surface tension we see when a drop of water forms a domelike shape on a surface.
- Water’s polarity also enables the capillary action that occurs when water-based fluids are pulled through tiny plant roots and blood vessels.
- Water is called the “universal solvent” because its polarity strongly attracts other charged or polar particles. By attracting positive ions to its negative end or attracting negative ions to its positive end, it dissolves more substances than any other liquid. But it cannot dissolve nonpolar or noncharged substances, like oil.
- Water is a special liquid—and ice is a special solid.
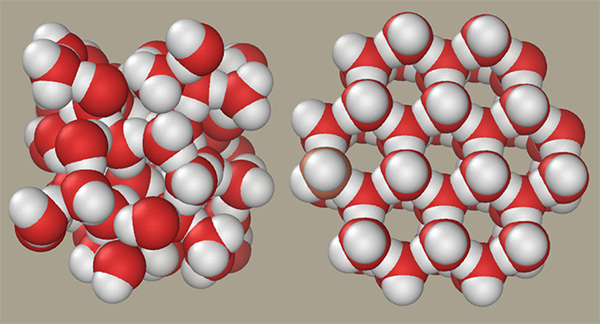
- For most substances, solids are denser than their liquid phase because they form more tightly packed frameworks of molecules as they cool, so they sink.
- But ice cubes and icebergs float; they are less dense than liquid water.
- When water freezes, the hydrogen bonds that organize the H2O molecules into structured ice crystals force additional space between the clumped-up molecules of liquid water.
- This additional space makes a cubic foot of ice weigh about 92 percent of the weight of a cubic foot of water. (A cubic foot of ice weighs 57.2 lbs (25.9 kg), more than 5 lbs less than a cubic foot of water, which weighs 62.4 lbs (28.3 kg).)
- There are only a few other elements whose solid form floats in the liquid form: silicon, germanium, gallium, arsenic, bismuth and plutonium.
- While audiences have long marveled at the elegance and agility of figure skaters or the power and speed of hockey players, scientists have long puzzled over how a skater gets started gliding on the ice in the first place.
- In the mid-1800s, scientist Michael Faraday observed that a thin layer of liquid water coats solid ice even at temperatures far below its freezing point, making ice slippery and influencing chemical reactions on icy surfaces.
- Faraday showed the effects of the water layer with his experiments, but the layer itself is so thin that it is virtually impossible to see with the human eye, and the science to explain the observation at a molecular scale had not yet been developed.
- You can see this process at work when ice cubes stick together, the thin water layers on each cube freeze together.
- A few years later, engineer and physicist James Thompson discovered that applying pressure melts ice.
- You can cut ice into blocks very slowly by putting weights on a wire stretched across the surface to increase the pressure along the wire. Once the wire passes through, the cut faces of the ice re-freeze.
- However, this doesn’t solve the mystery of how skaters can get going on ice, because the pressure of a skater is less than 1 percent of the pressure needed to melt the ice below him or her, and it couldn’t melt ice instantaneously enough for the skater to move across it.
- In 1987, the existence of Faraday’s “quasi-liquid” layer was proved with X-ray imaging.
- The layer ranged from 1 to 94 nanometers thick—1,000 times smaller than bacteria.
- Recently researchers have shown that this liquid coating is just 1 molecule thick in temperatures as cold as -36oF (-38oC)—well below the freezing point of water.
- They also showed that a step-change occurs as the ice warms to 3oF (-16oC) as a second layer of water molecules becomes mobile.
- How does the quasi-liquid layer form?
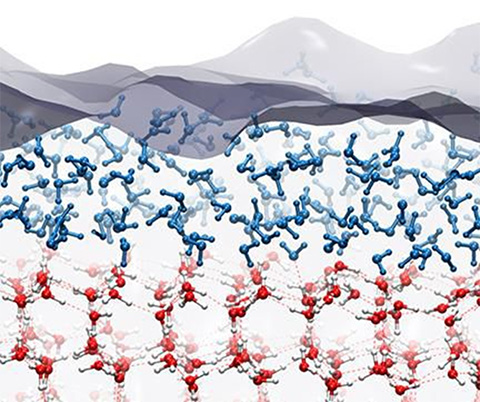
Solid ice is slippery because of a “quasi-liquid” layer at the surface. New studies with both computer models and direct measurements show that this layer forms as single layers of the ice crystal melt in a stepwise manner. Credit: Computer model image by Davide Donadio, UC Davis - When water is frozen, individual water molecules use hydrogen bonds to grab onto each other, creating the highly organized crystalline structure with four bonds.
- But molecules on the edges of the ice have air on one side so they can’t grab other water molecules on that side; they bond loosely with two to three hydrogen bonds.
- These surface molecules become disorganized trying to maximize their hydrogen bonds, diffusing across the surface like tiny ball bearings and making ice slippery.
- Some researchers think of it as a 2D layer of gas instead of a 3D layer of liquid.
- So, how do skaters get going? Skaters hydroplane on a thin channel of water that forms the instant their blades carve the ice.
- Faraday’s “quasi-liquid” layer gets the skate going instantaneously.
- As skaters plow through the ice, their skate blades create friction that melts tiny amounts of ice to supplement the surface liquid layer,
- Additionally, the skater’s blade deforms the solid ice structure, causing its molecules to disorganize into minute quantities of liquid water.
- Ice temperature impacts skating, so skaters adjust the radius of the blades on their skates to fine-tune their skating. The ice used in Olympic rinks is purified water, sprayed on rinks one layer at a time to create surfaces of flawless consistency.
- Ice rinks typically keep their ice at 24-25oF (-5 to -4oC) for general skating.
- 17-23oF (-8 to -5oC) is considered good for hockey, allowing for faster skating and fewer ice shavings, resulting in a smoother playing surface that allows the puck to slide more easily.
- 24-29oF (-5 to -2 oC) is the best temperature for figure skating, because it enables skates to bite deeper into the ice for greater control and better maneuvering, and it allows for softer landings.
- It would be hard to imagine skating at extreme low temperatures like -36oF (-38oC). Skating on a 1-molecule-thick water layer would limit the amount of hydroplaning and make skating much more difficult as temperatures dive.
- There is a point when ice loses its slipperiness; that’s for a future episode.
- Understanding how the structure of ice surfaces behaves under different conditions helps researchers to better understand how ice may influence chemical reactions on Earth and in the atmosphere.

