
Credit: Marie-Lan Taÿ Pamart (CC BY)
Phoenician soldiers, when injured in battle, would shave bronze from their swords into their wounds. Why? For the copper.
Copper is often found in its pure state, rather than bound in ore, and is easy to bend. This made copper the first metal to be shaped by humans into useful implements around 10,000 years ago.
The Copper Age lasted 5,000 years, until someone realized they could add tin to copper to make bronze, which was easier to melt and cast, harder as a finished product, and better for tools and weapons like Phoenician swords.
Those soldiers and their contemporaries knew that copper is antimicrobial. High-copper alloys, like bronze, preserve that attribute.
Egyptian doctors used copper to disinfect. Anatolian mothers fed babies from copper cups, since it killed the germs that caused diarrhea.
Modern researchers have finally caught on, and for the past 20 years have studied copper’s antimicrobial effects.
The process is still not completely understood, but when microbes—including the coronavirus—land on copper, it releases charged particles that invade the microbe, disrupt its DNA, and kill it within hours, even minutes.
Studies have found that hospitals can reduce infections by up to 10 times by replacing plastic and stainless steel with copper alloys, and many have begun to use them for high-touch surfaces.
The future of healthcare may benefit from the ancient properties of copper.
Background
Synopsis: Shiny, reddish copper was the first metal manipulated by humans, and it remains an important metal in industry today. Copper was one of the first metals ever extracted and used by humans, and it has made vital contributions to sustaining and improving society since the dawn of civilization.
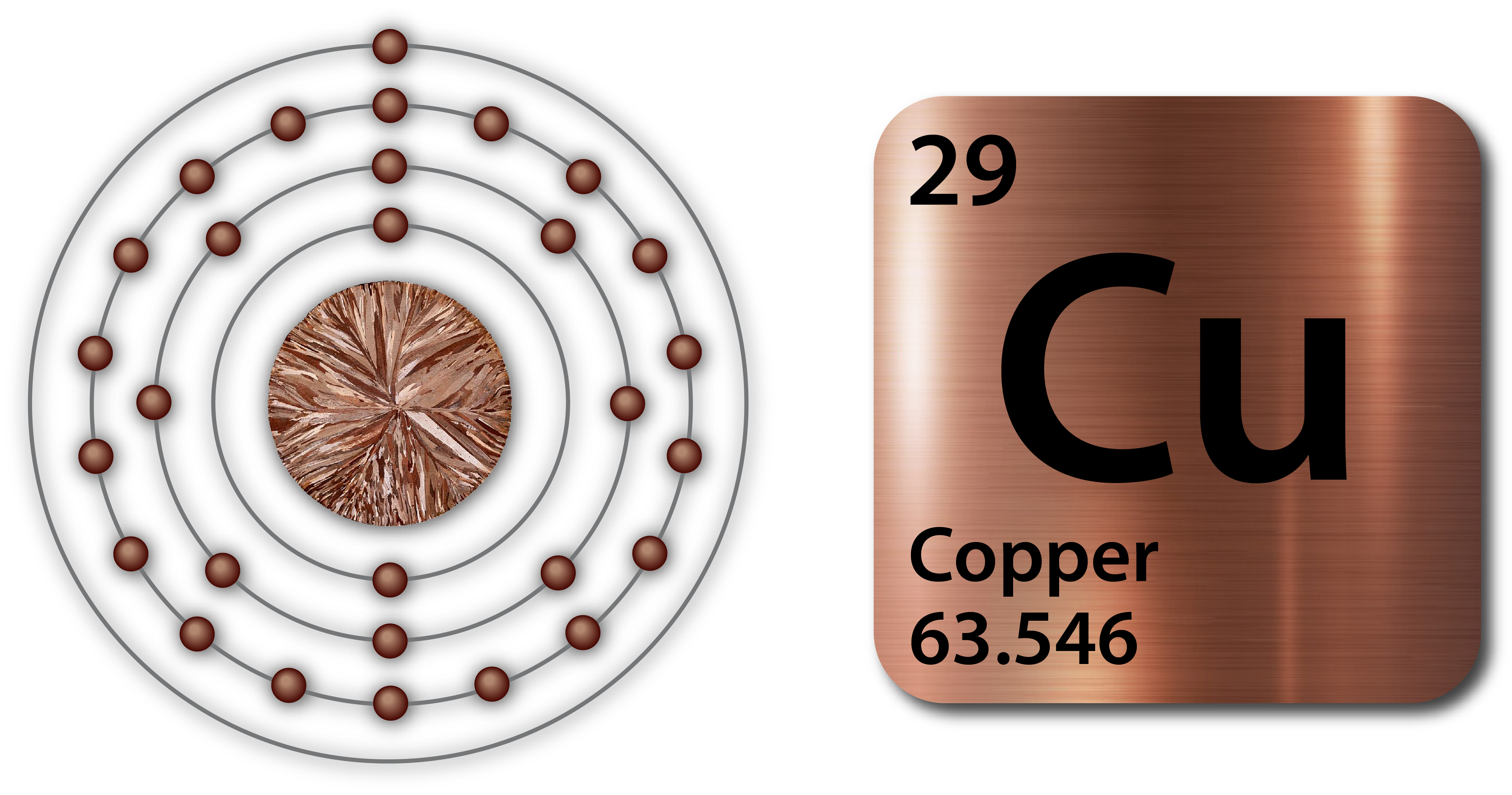
- Copper is a shiny, red-orange metal that is ductile, malleable, and soft.
- It has the atomic symbol ‘Cu’ and an atomic number of 29.
- It is the only pure metal besides gold that is not silver or gray.
- It has very high thermal and electrical conductivity.
- There are more than 160 copper-bearing minerals like turquoise, malachite, and azurite, many of them are green, blue-green or blue in color.
- Mollusks and some arthropods, like the living-fossil horseshoe crab, have copper-based blood that is blue.
- Copper salts like cupric sulfate and cupric chloride make fireworks that are green or blue, respectively.

Credit: Elcobbola (public domain)
- Copper weathers to a brownish color in air, but in the presence of air and water, copper loses electrons and oxidizes, forming a characteristic greenish copper oxide deposit on its surface. This layer is called “verdigris.”
- The Statue of Liberty is coated in a super-thin layer of greenish copper oxide that is only 0.005 in (0.127 mm) thick but weighs about 80 tons (73 metric tons).
- It took 34 years for the statue to change from copper-colored to verdigris, finally attaining its current verdigris luster in about 1920.
- Because copper is the metal most likely to be found in its native state, and because it can be easily shaped, stretched, and molded, it was one of the first metals to be used by ancient people.
- The word “copper” comes from the Latin cuprum, which was derived from Cyprium aes, meaning “a metal from Cyprus.” The island of Cyprus is where metallic copper was mined in ancient times.
- Copper has been used for nearly 10,000 years. The Copper Age occurred between the Stone Age and the Bronze Age.
- Copper beads dating from 8000 BC have been found in Turkey, and copper tools excavated in Israel date to as early as 5100 BC.
- Slags and crucibles from ca. 4500 BC indicate smelting was occurring at that time in Turkey, Bulgaria, Serbia, and Greece.
- In ca. 3300 BC, tin was added to copper to produce bronze, which is harder than copper and easier to melt for casting. Bronze is also harder and more corrosion resistant than iron, so it was better for making weapons.
- Coins have been made from copper since ca. 8000 BC. The U.S. penny was made of solid copper from 1783 to 1837. Varying amounts of tin and zinc were then added in stages until 1982, when the penny was reminted as a mainly zinc coin (97.5%) with a thin copper coating (2.5%).
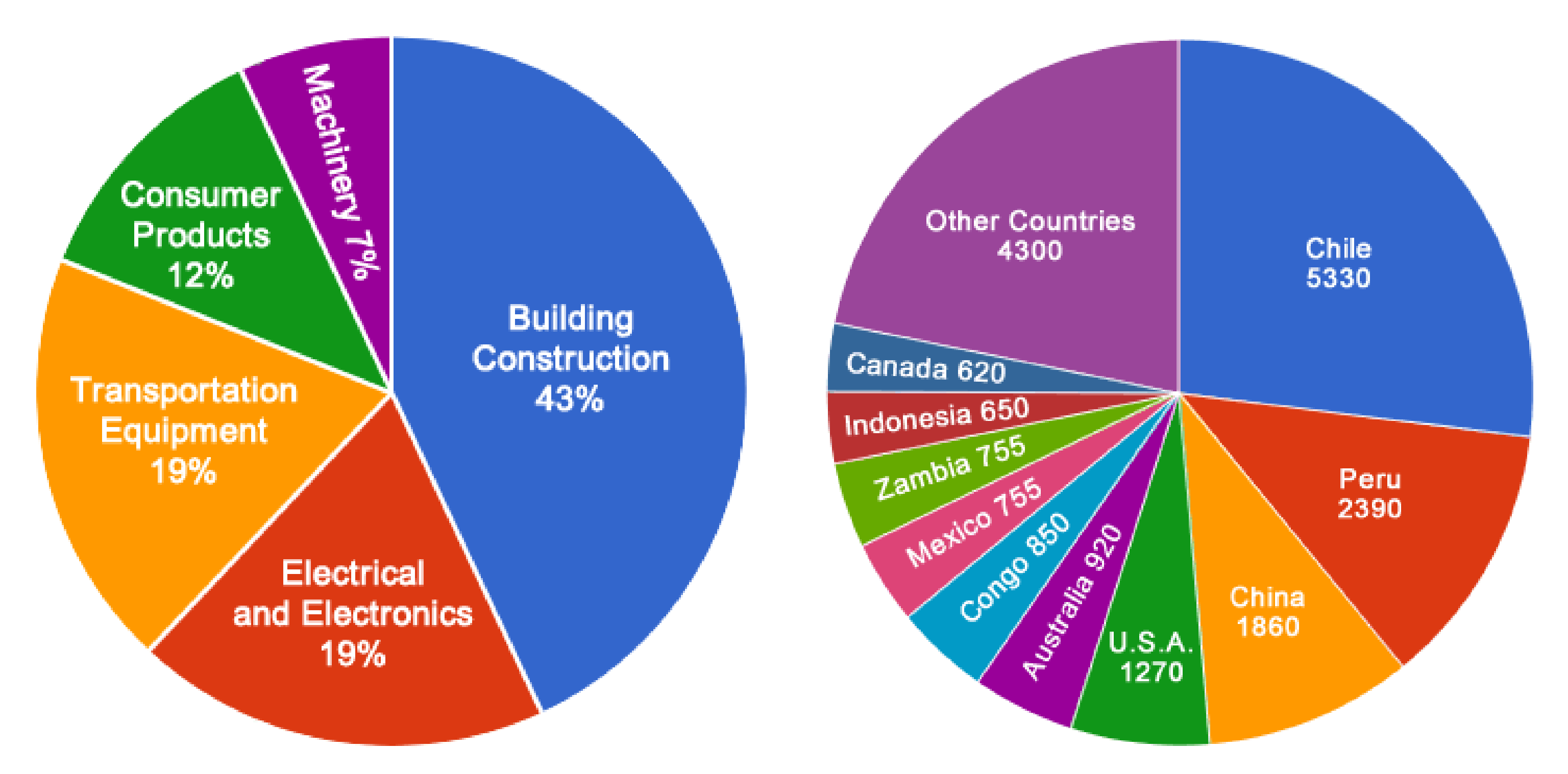
Credit: USGS
- After iron and aluminum, copper is the third most used industrial metal in the world.
- With its outstanding thermal and electrical conductivity and corrosion resistance, copper is used in building construction, power generation and transmission, and the manufacturing of electronic products and motors.
- Automobiles contain 45–100 lb (20–45 kg) of copper. There is almost 1 mi (1.5 km) of copper wiring in an average car.
- Copper has many useful alloys, or mixtures with other metals. When copper is alloyed with nickel as cupronickel, it forms a corrosion-resistant and anti-fouling surface for ship bottoms. In trumpets, trombones, and cymbals, the copper-zinc alloy known as brass produces higher-quality acoustics.
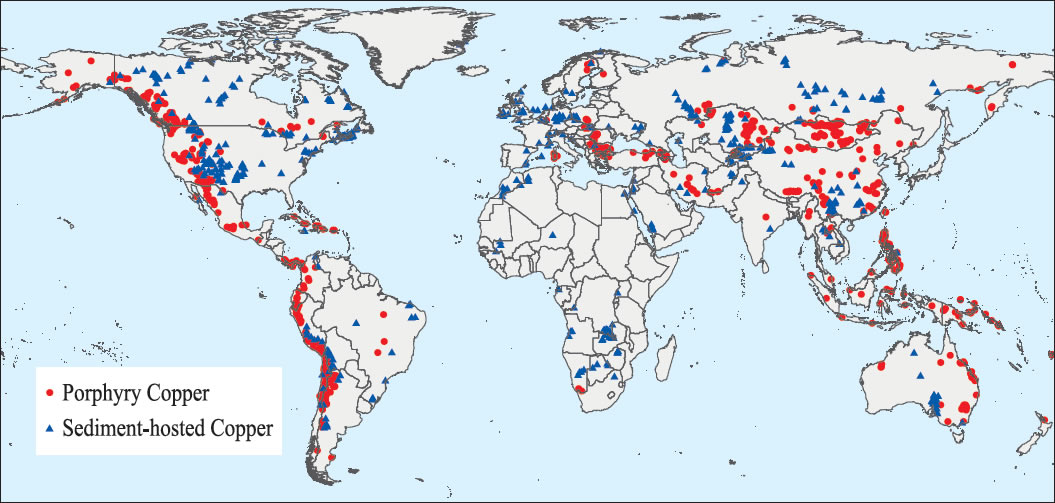
Source: USGS
- More than two-thirds of the copper deposits on Earth occur as porphyry copper found in igneous rocks, while more than one-fourth are found in sedimentary rocks.
- Chile is the highest producer of copper in the world, followed by Peru, China, and the United States.
- In the United States, Arizona produces the most copper, followed by Utah, New Mexico, Nevada, and Montana.
- Copper is essential for both plant and animal life, but too much can be toxic.
- Copper is a trace metal that is necessary for forming red blood cells
- Food sources of copper include seafood, grains, beans, nuts, potatoes, whole grains, leafy greens, and chocolate.
- High levels of copper can cause jaundice, vomiting, abdominal pain, diarrhea, anemia, and convulsions.

Credit: C.G. Cunningham, USGS
- In the past couple of decades, scientists have been studying another magical property of copper—it is antimicrobial.
- In 2020, researchers found that the virus responsible for COVID-19 survives for days on most surfaces, but on copper, 96% of it dies after just 2 hours (99.5% after 5 hours). This was not a big surprise to microbiologists who had been studying copper’s microbe-killing power for decades.
- Copper-based metals have been shown to destroy 99.9% of dangerous microbes—like E. coli, MRSA, Staphylococcus, swine flu (H1N1), C. difficile, Influenza A, adenovirus, fungi, and one of the coronaviruses that causes the common cold and pneumonia—in just a few minutes to 2 hours.
- Copper and copper alloys have been used in hospitals for high-touch surfaces like bedrails, call buttons, and doorknobs, and can even be woven into socks to kill foot fungus. A recent study showed that two-thirds of copper-plated stethoscopes in a medical ward were completely microbe free.
- The U.S. EPA lists more than 400 types of copper surfaces as “antimicrobial materials with public health benefits.” One in every 31 U.S. hospital patients gets a hospital-based infection, and copper surfaces have repeatedly been shown to reduce these infections, saving both costs and lives.
- Copper lasts a long time. One-hundred-year-old railings in New York City’s Grand Central Station are just as antimicrobial today as the day they were installed.

Credit: Metropolitan Museum of Art
- Ancient people knew about copper’s superpower millennia ago.
- An ancient document known as Smith’s Papyrus records the earliest known reference to copper as an infection-killing solution by an Egyptian doctor in 1700 BC.
- In China, copper coins were used as early as 1600 BC to cure heart, stomach, and bladder diseases.
- The Phoenicians sprinkled shavings from their bronze swords into their wounds to fight infection.
- Copper drinking vessels have been used for millennia to prevent diarrhea in children.
- Many metals are known to be antibacterial, but copper is superpowered thanks to its atomic configuration.
- Unlike gold or silver, copper has a free electron in its outer shell, making it very reactive.
- Like a molecular grenade, it punches holes in cell membranes and viral coatings, blowing apart bacteria, fungi, and viruses and destroying the DNA and RNA of microbes so they can’t mutate.
- Copper surfaces should still be sanitized as usual, but copper never loses its inherent antimicrobial disinfectant capabilities. Even tarnished copper kills microbes.
- Recently, Chile’s Minister of Mines suggested incorporating copper into the fabric of medical masks to protect wearers. It’s an idea worth testing!

