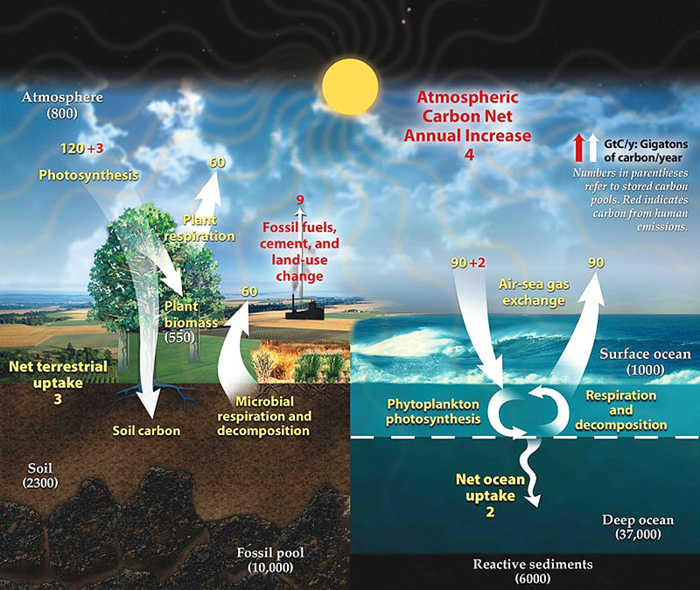
Credit: Diagram adapted from U.S. DOE, Biological and Environmental Research Information System. Public domain, via Wikimedia Commons.
All known life on Earth is carbon based. Today, though, when we hear about carbon, it’s usually in terms of emissions. Or the idea of capturing and storing those emissions.
What you may not have heard is that Earth has been emitting and storing carbon for millions of years, cycling it between sky, sea, soil, and rock.
Deep in the geologic past, atmospheric carbon dioxide was 10 times higher than today. Then ancient ocean life began to use it.
Early marine organisms used CO2 dissolved in seawater for photosynthesis, forming the base of the food chain. Other organisms used it to build their exoskeletons and shells.
When they died, their carbon-rich remains sank to the seafloor and were buried. Very slowly, carbon was being stored within the earth.
Millions of years later, plants evolved and dramatically changed the carbon balance. They began to turn huge volumes of atmospheric carbon into organic carbon like carbohydrates and cellulose.
Some organic carbon goes back into the atmosphere or the soil. Some gets buried and becomes part of sedimentary layers and, with enough pressure and time, can be cooked into hydrocarbons—fossil fuels.
Today, the burning of fossil fuels is moving ancient carbon stored in the earth back into the atmosphere. Researchers are studying different ways to sequester that CO2, and we’ll talk about that on a future EarthDate.
Background
Synopsis: When we burn fossil fuels like coal, oil, and gas, we release carbon, stored away for millions of years below the surface of Earth, as it reacts with atmospheric oxygen. Many other activities of modern life also impact the delicate balance of gases in our life-sustaining atmosphere. As we study ways to mitigate changes to the atmosphere, what can we learn from the natural carbon sequestration that goes on constantly on Earth’s surface?
- What does it mean to sequester something?
- Sequestering is defined as isolating and protectively storing something away.
- In chemistry, that means storing it where it can’t react (like placing lithium in mineral oil) or forming a stable compound that makes it unavailable for other reactions (quartz is formed from silicon and oxygen).
- How does Earth naturally sequester carbon?
- Carbon is essential for life. Everything living contains carbon, including you—you are about 18% carbon! You store and cycle carbon through your body until you die.
- Plants extract carbon dioxide from the atmosphere in order to manufacture sugars during photosynthesis. They deliver the sugars to microbes in the soil, which feed the root system of the plant.
- Animals absorb carbon when they eat plants, or when they eat other animals that have consumed plants.
- A byproduct of photosynthesis is oxygen, lucky for us!
- When plants and animals die, they decompose and return their carbon to the soil.
- Erosion delivers carbon-rich material to rivers and deltas and, finally, to the sea. Organic-rich materials and sediments in deltas are buried rapidly.
- The oceans are amazing reservoirs for carbon.
- Seawater has a remarkable buffering capacity to absorb and store dissolved CO2, but abrupt changes in concentration of CO2 in the ocean can disrupt food chains.
- Marine organisms use CO2 for photosynthesis and for building their shells. When marine organisms die, they rain down to the seafloor, where they are preserved in rock layers.
- Earth’s crust sequesters a substantial amount of carbon in both inorganic carbonate rocks like limestone and organic-rich mudstone and shale.
- Organic-rich layers are converted into coal, oil, or gas as they are subjected to the high pressures and temperatures of burial through geologic time. Buoyant hydrocarbon fluids migrate through pores and cracks in rocks until they hit an impermeable barrier and are trapped.
- Burial keeps the carbon sequestered for many millions of years, until we release it by burning fossil fuels.
- As sediments riding on plates are subducted along plate margins, fossilized organic carbon makes its way into the deep crust and upper mantle.
- Some of this carbon reemerges as CO2 from volcanic eruptions, and some of it is metamorphosed into graphite as a result of increasing heat and pressure.
- Recently, scientists have shown that some volcanic rocks called rhyolites, which produce CO2-rich explosive volcanic eruptions, can only carry so much carbon back to the atmosphere in their magma; the balance ends up deep in the mantle, isolated from surface chemical reactions—possibly for eternity.
- Earth’s sequestration of carbon in thick sedimentary basins may have contributed to the increase of oxygen in the atmosphere that enabled explosions of life.
- Current research has correlated rapid deposition and burial of sediments both 2.3 billion years ago and 500 million years ago with atmospheric oxygen peaks.
- Ongoing research about when plate tectonics and subduction started on Earth may provide insight into why these basins formed and when fossil carbon was first subducted into the mantle.
- Mantle carbon is the source for diamond formation below the deep keels of the continents, as we explained in a previous episode of EarthDate.

