
Credit: Peggy Greb, U.S. Department of Agriculture, public domain, via Wikimedia Commons
Background
Synopsis: Rare earth elements (REEs) are needed for many current technologies yet are difficult to mine directly. New data reveals that we might be able to obtain these important resources from waste.
- Rare earth elements are not necessarily rare, they are instead weakly concentrated and dispersed in host rock such that it takes a tremendous amount of mining to produce enough REE material to matter.
- REEs consist of seventeen elements from the periodic table that include the group called the lanthanides along with elements scandium and yttrium.
- All the elements in the lanthanides share a similar arrangement of electrons. It is this unique pattern of electrons that contributes to the unique chemical and physical properties of these elements.
- Rare earth elements are renowned for strong magnetic properties. They have many unpaired electrons that impart this important feature.
- As a result, REEs are used in the powerful magnets needed for MRI machines, electric motors and magnetic resonance spectrometers (MRS).
- MRS complements MRI by providing information about the biochemical composition of tissue. This allows for a “virtual biopsy” that would aid in detecting brain tumors, strokes and Alzheimer’s disease and to study organs such as muscles and liver.
- REEs also exhibit uncommon optical properties. Rare earth elements like europium and terbium can emit specific colors of light when energized.
- This makes them useful in phosphors for fluorescent lights, TV screens and computer displays.
- The distinct electron structures of rare earth elements give them predictable chemical behavior, allowing them to form stable compounds.
- This makes them useful in catalysts for industries like petrochemicals, metallurgy, electronics, health care and environmental technologies.
- Rare earth elements have larger atoms, which help them form stable compounds in crystal structures.
- This makes them important for creating alloys and ceramics used in electronics and high-temperature settings.
- REEs are integral into many of the electronics that we use daily including laptops, tablets and smartphones.
- The unique characteristics of REEs contribute to the compact size, performance and energy efficiency of these devices.
- Because of their unique characteristics, they do not have proper substitutes, making them essential for production but also vulnerable to supply chain issues.
- Some specific examples of rare earth elements are:
- Lanthanum (La): used in batteries, glass, lighting systems and alloys
- Neodymium (Nd): key component in strong magnets used in electric motors, wind turbines and hard drives
- Europium (Eu): used in florescent lighting and some screens
- Gadolinium (Gd): used in MRI contrast agents
- Yttrium (Y): used in high-temperature superconductors and other applications
- The rare earth metals are thus essential for modern technologies.
- Currently the U.S. relies mostly on imports of these important elements, especially from China.
- These elements are not “rare” on Earth but are not usually found in large deposits. Instead, they must be extracted or refined from other ores.
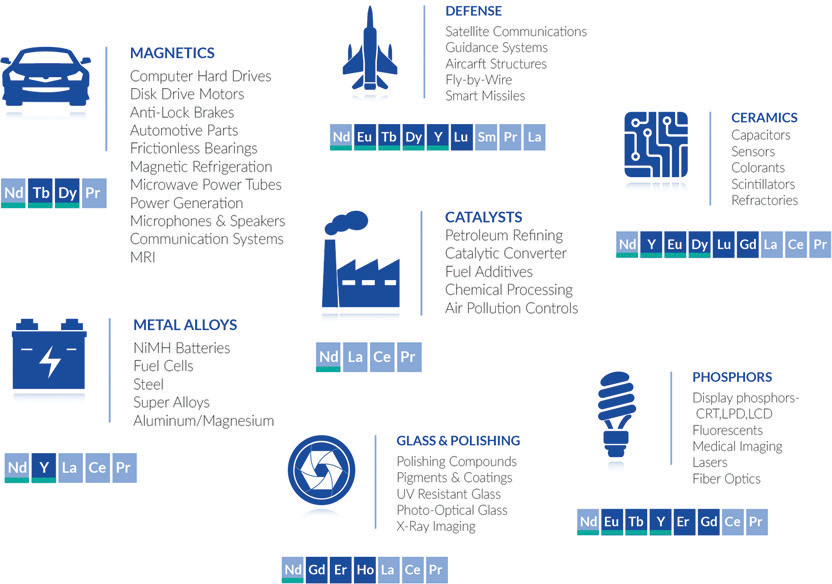
Rare earth elements are essential to modern life, playing a key role in many everyday technologies.
Credit: National Energy Technology Laboratory
- Coal ash, a waste product from burning coal in power plants, contains concentrated amounts of rare earth elements making it a promising domestic resource for these critical materials.
- When we think about burning coal, carbon dioxide emissions usually come to mind first. However, combustion also produces another significant byproduct: coal ash.
- At every coal-burning power plant across the globe, coal ash is created during the combustion process. Over the decades, millions of tons of this ash have accumulated in landfills and surface ponds, often located near power plants.
- These storage sites are considered environmental hazards. If not carefully managed, coal ash can release harmful metals like mercury, cadmium and arsenic, which can leach into the soil, groundwater or nearby rivers. The ash can also become airborne, contributing to air pollution and threatening public health.
- A dramatic example of this occurred in Kingston, Tennessee, in 2008, when a coal ash storage pond collapsed. Over 5.4 million cubic yards of ash were released, spreading across hundreds of acres and contaminating the Emory River.
- Despite these risks, scientists and engineers are rethinking coal ash. What has long been seen as both an environmental burden and an industrial waste product may, in fact, become a valuable source of critical materials—especially rare earth elements used in energy technologies, industry, health care and electronics.

In 2008, the largest coal ash spill in U.S. history occurred when a containment dam at the Kingston power plant in Tennessee failed, releasing 5.4 million cubic yards (about 1.1 billion gallons) of coal ash into nearby rivers and surrounding land.
Credit: Brian Stansberry, CC BY 3.0, via Wikimedia Commons
- At the Bureau of Economic Geology at The University of Texas at Austin, Dr. Bridget Scanlon and her team combed through vast amounts of governmental and academic records, building the most complete picture yet of where coal ash is stored and what’s in it.
- Instead of digging into the ground, they dug through data.
- They discovered that millions of tons of ash could contain economically significant levels of rare earth elements—vital for everything from electric vehicles to satellites.
- They confirmed that there is a lot of coal ash in the United States.
- Between 1950 and 2021, the U.S. burned 51.7 billion tons of coal, producing about 5.3 billion tons of ash, around 10% of coal’s original weight.
- Roughly 70% of that coal ash is still sitting in landfills and ponds, making it potentially accessible for REE extraction.
- But the Bureau team also recognized that not all the coal ash is the same and coal from different sources contains differing amounts of REEs, some requiring more effort to extract the valuable elements.
- Coal ash from the Appalachian Basin contains the highest average concentration of REEs, approximately 431 mg/kg.
- Despite the high concentration, only about 30% of these elements can be extracted using standard methods. This is because the REEs are often trapped within glassy mineral structures formed during coal combustion, making them less accessible to common extraction techniques like nitric acid leaching.
- Coal ash from the Illinois Basin has a moderate average REE concentration of around 320 mg/kg.
- The distribution of REEs in Illinois Basin coal is uneven, with higher concentrations found near certain geological features like sandstones. This variability can affect the efficiency and consistency of extraction processes.
- Coal ash from the Powder River Basin in Wyoming contains a lower average concentration of REEs, about 264 mg/kg.
- Despite the lower concentration, up to 70% of REEs can be extracted from Powder River Basin coal ash. This is due to the simpler chemical composition of the ash, which lacks the complex glassy matrices found in Appalachian coal ash, making REEs more accessible to extraction methods.
- If REEs were extracted from all this available ash, the potential market value could be as high as $8.4 billion—and even more (up to $97 billion) if elements like yttrium and scandium are included.
- Extracting REEs from coal ash could also help reduce environmental hazards, especially from unlined landfills and ash ponds that can contaminate groundwater.
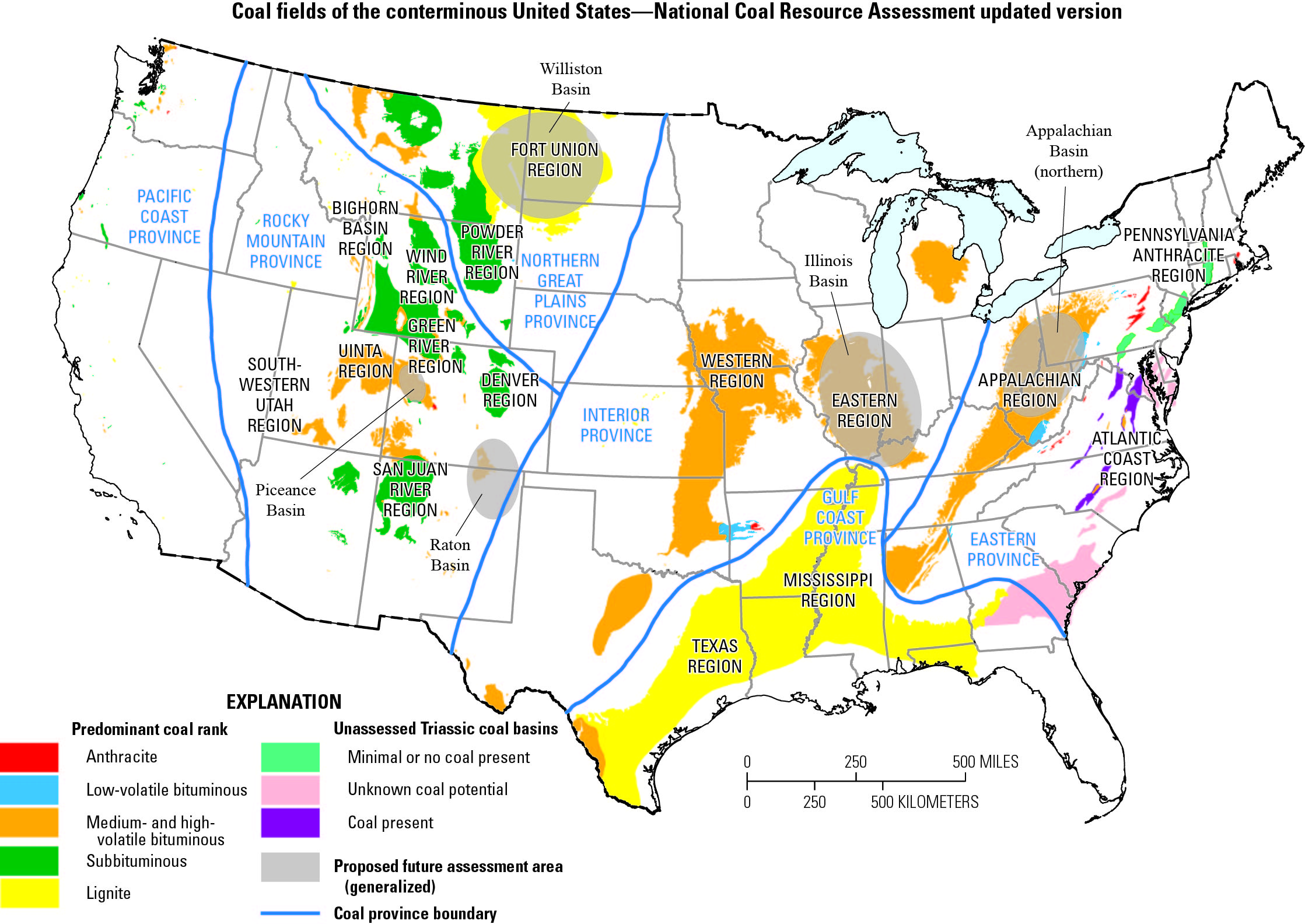
This map shows major U.S. coal fields. Ash from Appalachian and Illinois coals has more rare earth elements, but they’re harder to extract. Powder River Basin coal has fewer rare earths, but they’re easier to remove.
Credit: U.S. Geological Survey, public domain
- Coal ash from the Appalachian Basin contains the highest average concentration of REEs, approximately 431 mg/kg.
- The work from Dr. Scanlon and the Bureau of Economic Geology encourages the development of pilot projects to test extraction technologies. These could occur in the U.S. but also a great strategy internationally in coal-heavy regions like China, India and the European Union.
- Pilot projects help determine whether rare earth element extraction from coal ash is technically effective and economically worthwhile.
- They support the transition from small-scale lab experiments to full industrial operations.
- These projects can reduce the environmental risks of coal ash disposal by turning waste into usable materials.
- They offer the potential to create new industries and jobs, especially in areas impacted by the decline of coal-based energy.
- Dr. Scanlon’s work sets the stage for this encouraging action by providing a map, database and starting point. Engineers, policy makers and environmental scientists need to figure out how to make this a reality. In doing so, cleaning up waste and building a greener future might be the same project.
Episode script
Rare Earth Elements, or REEs, are a group of 17 trace metals that play important roles in the electronics we rely on daily. Their properties are so unique, they’re not replaceable.
But their current supply is almost completely controlled by China, which creates shortages and security challenges. So, researchers are looking for domestic sources—and have found a surprising one: coal ash.
When coal is burned at a power plant, it’s reduced to gases and about 10 percent of its weight in ash. In that ash, the trace metals from the coal become concentrated.
Scientists from the Bureau of Economic Geology first recognized that coal ash could be a source for REEs.
When they surveyed the ash produced by U.S. coal plants since 1950, they found 35 million tons that could be available for REE recovery. It’s currently stored in landfills and retaining ponds, mostly near the power plants.
There are different concentrations of REEs in the ash. Eastern coal has the highest concentration, and Western coal the lowest—but western coal’s simpler molecular structure could allow more REEs to be recovered.
The next step is to set up pilot projects to test the process, its energy requirements and environmental impacts.
The preliminary studies look promising, offering a chance to build a domestic industry in Rare Earth Elements, while building better long-term storage for the coal ash. A technology win-win!

