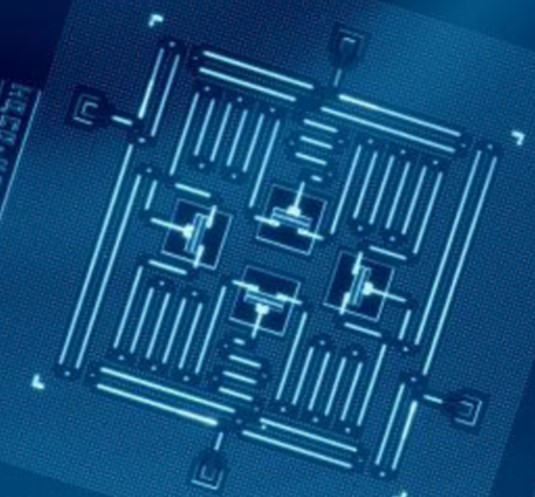
Credit: Gambetta et al. (2017), CC BY 4.0, via Wikimedia Commons
Background
Synopsis: In 1925, Werner Heisenberg helped launch the field of quantum mechanics, revealing the strange and unpredictable nature of the atomic world. A century later, his discoveries are driving breakthroughs in technology and transforming our understanding of Earth and the environment.
- Imagine a young physicist in 1925, staring at the puzzling behavior of tiny particles inside an atom. His name was Werner Heisenberg, a German scientist fascinated by the fundamental building blocks of the universe.
- At the time, scientists believed they could measure everything precisely including an electron’s position, its speed, and its path, just like the planets orbiting the Sun.
- But as Heisenberg studied electrons, he discovered a major problem, and the numbers just didn’t add up.
- Heisenberg realized that certainty had its limits in that you couldn’t measure both an electron’s position and speed perfectly at the same time.
- This wasn’t just a strange quirk of physics—it meant that at the atomic level, what was thought to be reality was unpredictable.
- Heisenberg’s work laid the foundation for quantum mechanics, a new field that would revolutionize science. But at the time, even he didn’t fully grasp how world-changing his discovery would become.

Heisenberg’s uncertainty principle states that we cannot precisely know both the position and momentum (speed and direction) of a particle at the same time. The more accurately we measure one, the less accurately we can know the other. This isn’t for lack of necessary measuring tools; it’s just a fundamental limit of nature.
Imaging trying to take a perfectly clear photo of a speeding car at night. If you use a fast shutter speed, you get a sharp image of the car (you know its position), but the headlights are just frozen, so you can’t tell how fast it’s moving. If you use a slow shutter speed, the headlights blur into streaks (showing motion), but now you can’t pinpoint exactly where the car is. The same trade-off applies at the quantum level with particles like electrons.
Credit: YudDy Yu, CC BY-SA 4.0, via Wikimedia Commons
- Heisenberg’s breakthrough in 1925 kicked off a century of astonishing discoveries in quantum science.
- The United Nations has declared 2025 as the 100th anniversary of quantum mechanics, recognizing its impact on modern technology.
- Some of the biggest milestones along the way:
- 1927 - Schrödinger's Cat: Erwin Schrödinger proposed his famous thought experiment showing how quantum particles can exist in multiple states at once.
- 1935 – Einstein’s “Spooky Action” at a Distance: Albert Einstein was skeptical yet still helped describe quantum entanglement, where particles can be mysteriously linked across space.
- 1950s–1960s – Birth of Quantum Computing: Scientists started exploring how to use quantum mechanics to build powerful new types of computers.
- 1980s – Quantum Encryption: Researchers discovered that quantum properties could make unbreakable codes for secure communication.
- 1990s – First Quantum Algorithms: Scientists developed special programs that quantum computers could run, proving their advantage over regular computers.
- 2000s–Present – The Quantum Leap: The first real quantum computers were built, and now companies like Google, IBM, and startups worldwide are racing to develop practical quantum machines.
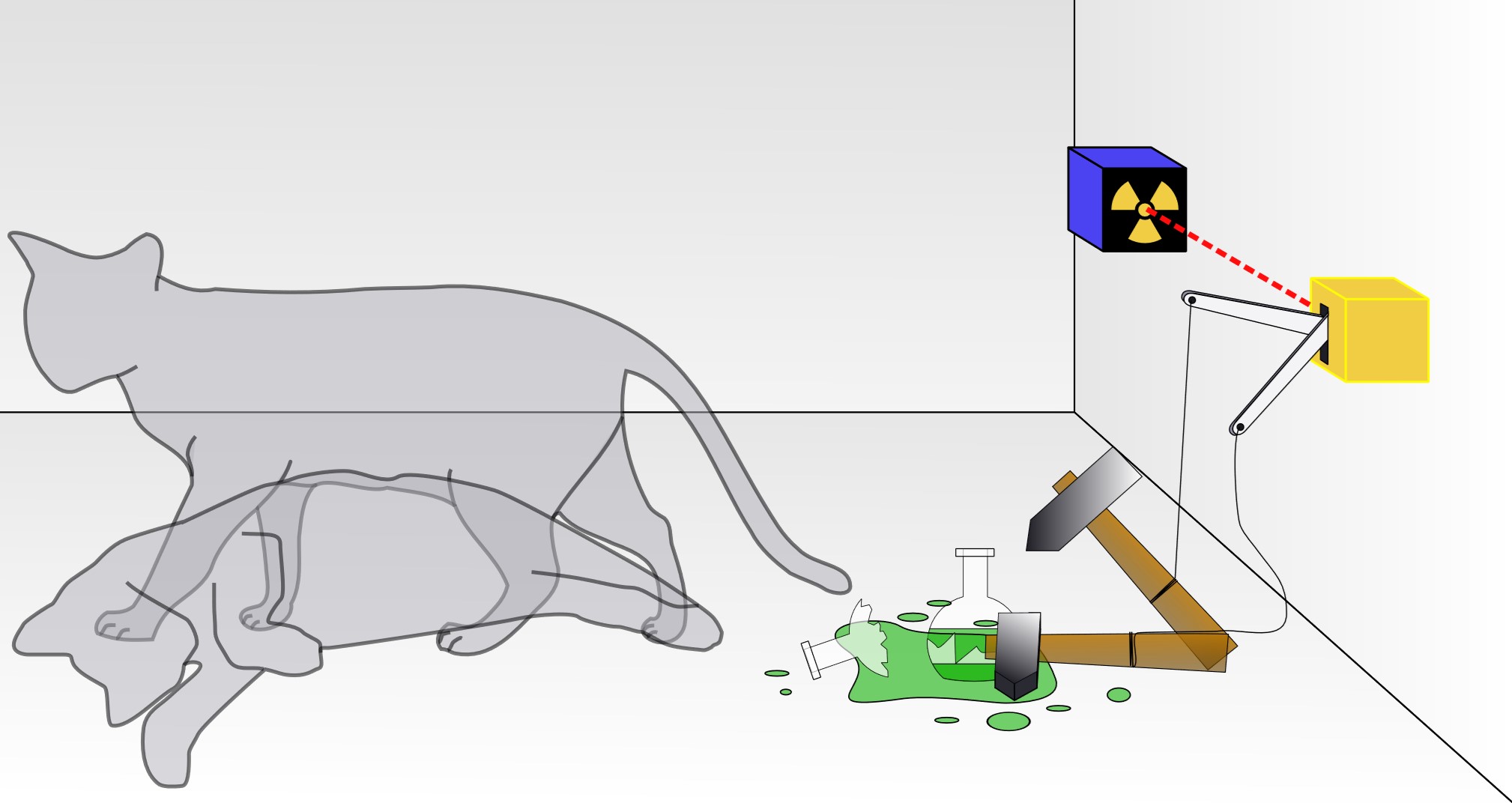
"Schrödinger's cat" is a thought experiment that illustrates superposition, where a quantum system exists in multiple states at once until it is observed. In the experiment, a cat is placed in a box with a radioactive atom that has a 50% chance of decaying and triggering a poison release. Until we open the box and check, the cat is both alive and dead at the same time in a quantum superposition. The moment we observe it, the cat’s state is determined. Another analogy would be flipping a coin and catching it in your hand without looking. While it’s covered, it’s both heads and tails in a sense—it hasn’t “decided” yet. The moment you look, you force it to take a definite outcome. In the quantum world, particles can stay in this uncertain state until they are observed.
Credit: Dhatfield, CC BY-SA 3.0, via Wikimedia Commons
- Today’s quantum computers take advantage of superposition and entanglement, two of the weird quantum properties, to solve problems in minutes that classical computers would take centuries to crack.
- Regular computers use bits (binary digits) as the smallest unit of data that a computer can process. A bit is always in one of two physical states, either 0 or 1.
- The processor in a 64MB iPhone is made of a silicon wafer composed of 512 bits.
- Quantum computers use qubits, which can be both 0 and 1 at the same time.
- This allows them to analyze enormous amounts of data simultaneously, making them ideal for solving Earth’s toughest challenges.
- Because of superposition and entanglement, a quantum computer uses far fewer qubits.
- A quantum computer may have only 32 qubits but due to entanglement, each qubit can interact with the each of the other qubits, creating 232 or 4.3 billion entangled states.
- When you also consider the added benefits of superposition, the result is an extremely powerful processor.

Entanglement is often referred to as a cosmic coin flip. Imagine you and a friend each have a mystery coin, but these coins are magically linked. You agree to take yours to New York and your friend takes theirs to Tokyo. Neither of you knows whether your coin will land on heads or tails—it’s totally random. But the moment you flip your coin, no matter how far apart you are, your friend’s coin instantly shows the opposite side! If yours lands on heads, theirs will always land on tails, even though they didn’t flip it themselves. It’s as if the two coins are secretly communicating faster than light! That’s quantum entanglement—when two particles are mysteriously connected, so that what happens to one instantly affects the other, no matter the distance.
Credit: ICMA Photos, CC BY-SA 2.0, via Wikimedia Commons
- A century after Heisenberg’s discovery, quantum mechanics is now helping to solve many complex problems.
- Quantum computers are tackling major environmental problems, such as:
- Carbon Capture: Designing better materials to remove CO2 from the air through enhanced design using quantum chemistry.
- Water Purification: Helping scientists create more efficient filters to provide clean drinking water.
- Energy Efficiency: Optimizing solar panels, batteries, and even nuclear fusion for better energy solutions including grid management and security.
- Climate Modeling: Simulating complex climate patters to better predict storms, heatwaves, and rising sea levels.
- What once seemed like strange, abstract science is now having an impact and providing sustainable solutions to complex issues.
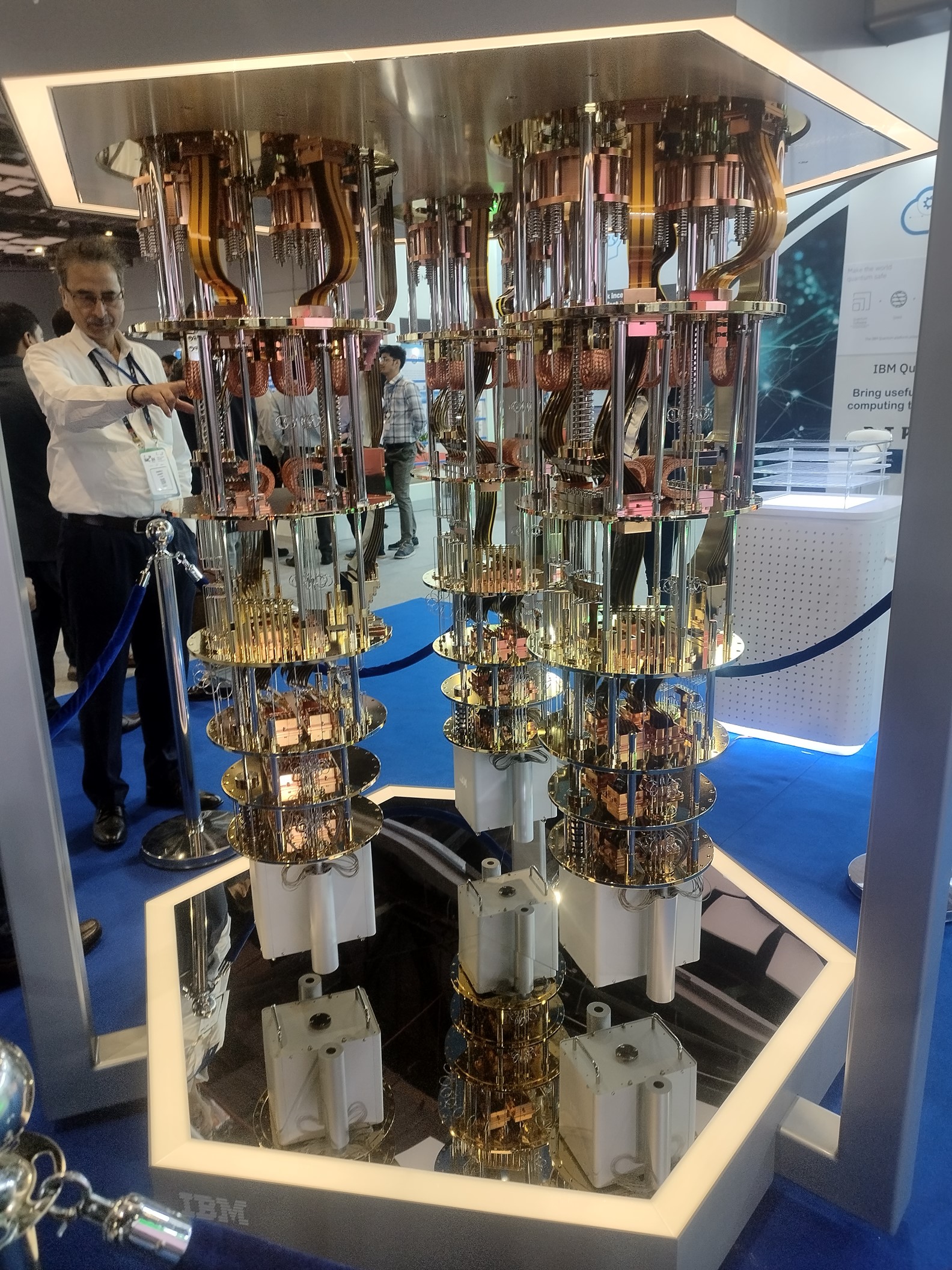
IBM had one of its quantum computers on display at the International Telecommunications Union conference, held in Delhi, India, in 2024. The processor, located at the very bottom of the device, is smaller than the palm of your hand, and must be kept at temperatures very close to absolute zero (minus 273 degrees Celsius). Many of the other components are needed to keep the machine cool and motionless. Industrial quantum computers cost in the millions of dollars.
Credit: Dev Jadiya, CC BY-SA 4.0, via Wikimedia Commons - If Heisenberg could see today’s quantum breakthroughs, he would be amazed beyond belief. His frustration with measuring electrons led to a century of world-changing discoveries.
- Quantum mechanics isn’t just about atoms anymore. It is building technology that is transforming the world.
- As we celebrate the 100th anniversary of quantum mechanics in 2025, we’re only beginning to see how powerful this science truly is.
- And if you’re still a little confused by quantum, you’re not alone. The renowned physicist Richard Feynman had this to say about the field: “If you think you understand quantum mechanics, you don’t understand quantum mechanics.”
Episode script
Imagine taking a photo of a speeding car.
A fast shutter freezes the car in position but gives no idea of its speed. A slow shutter produces a blurred streak, suggesting speed but not much about position.
This analogy applies to electrons. In the 1920s, German physicist Werner Heisenberg was studying them. The closer he came to measuring their positions, the less he could measure their speed. And vice versa.
He realized that speed and position are simply uncertain on the atomic scale. He called this the Uncertainty Principle, and it launched the field of quantum physics.
In the decades to come, things got weirder, as physicists recognized atomic particles could be in several places at once, called superposition, and entangled across space -- and time.
Computer scientists have now applied these concepts to experiment with quantum computing.
Normal computers use binary bits – which are either 0 or 1. In quantum computers, bits can be 0 and 1 … at the same time.
While expensive and complicated, quantum computers can process millions of times faster and will likely change what’s possible in data modeling and complex problem solving.
If you find all this confusing, you’re not alone. Even the renowned physicist Richard Feynman said: If you think you understand quantum mechanics, you don’t understand quantum mechanics.
I think I understand…

