You’ve probably heard that no two snowflakes are alike. And mostly, that’s true.
Snowflakes aren’t formed from freezing water, but when water vapor freezes, skipping the liquid stage altogether.
This allows the water molecules to organize themselves into the snowflake’s delicate structure.
Snow crystals come in more than 30 basic shapes, with the classic star-shaped snowflake forming best in temperatures around 5 degrees Fahrenheit.
These begin when water vapor freezes around a nucleus like a dust particle. This forms a single hexagonal ice crystal, which is heavier than air and so begins to fall.
As it does, the ice crystal grows. The original hexagon sprouts hexagons on each corner. These grow hexagons on their corners. And so on.
But the exact form that the growing snowflake takes is influenced by changing humidity and temperature levels in the air it falls through.
And because no two flakes follow the exact same path from sky to Earth, no two grow in the exact same conditions, and therefore no two are the same.
The only exception is when scientists make them in a laboratory, where they can control the conditions so precisely, they can produce identical flakes.
So if you find yourself out on a snowy day, take a moment to marvel at the thousands of snowflakes you can see falling, the millions in your yard, or billions in a field. Like people, no two are or ever will be the same.
Background
Synopsis: Snowflakes capture our imagination with their fleeting beauty and uniqueness. They are crystals of ice that form when water vapor in air freezes directly into ice, organizing itself into familiar intricate hexagonal designs. Snow crystals take many interesting forms that depend on the conditions in the cloud where they form.
- What are snowflakes?
- Snowflakes may be a single snow crystal or clumps of snow crystals.
- Sometimes snow crystals collide with water droplets in clouds, forming blobs that are called graupel, or soft hail.
- When raindrops freeze, they form sleet, not snowflakes.
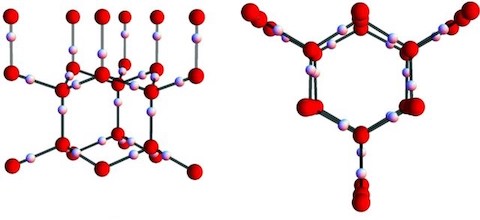
- Snow crystals don’t form from liquid water, they form from water vapor that freezes directly. The crystals never pass through a liquid phase, giving the molecules the opportunity to organize into specific crystalline structures.
- Ice is the solid form of water. Because it is a naturally occurring inorganic solid with a regular structure, it is considered a mineral.
- Ice crystals display flat, faceted surfaces just like other mineral crystals. It is these facets that sparkle in the sun when snowflakes fall or catch the light.
- Snow crystals occur in a variety of shapes with specific names.

- Snow crystal molecules tend to line themselves up into precise hexagonal arrays. Their forms have a hexagonal symmetry that reflects the normal crystalline structure of ice.
- Ice crystals are made from an ordered array of water molecules at the atomic scale.
- Even though snow crystals of the familiar star-like “stellar” type of look pretty flat, they are actually three dimensional.
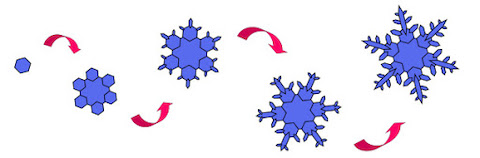
- Snowflakes form progressively as they fall through the atmosphere, and no two natural snowflakes look exactly alike.
- A stellar-type snow crystal begins when a small hexagonal plate of ice forms around a nucleus such as a dust particle.
- Branches sprout from the six corners as the crystal grows larger.
- As it tumbles through the clouds, the crystal experiences ever-changing temperatures and humidities, and each change makes the arms grow a bit differently.
- The exact shape of the final snow crystal is determined by the precise path it takes through the clouds.
- The six arms all grow independently but take the same path, so each experiences the same changes at the same times. Thus, the six arms grow in synchrony, yielding a complex yet symmetrical shape.
- No two snow crystals follow the exact same path through the clouds as they fall, so that is why no two snow crystals look exactly alike. But scientists can make identical snow crystals in the laboratory by exposing them to identical conditions.
- Most snow crystals are not perfectly symmetrical because of slight differences that occur during the formation of their branches.
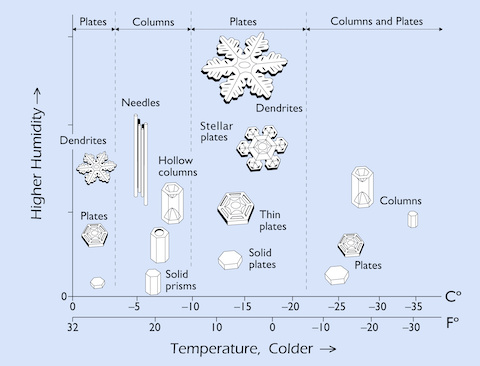
- The shape of a snow crystal depends on the temperature and humidity of its cloud surroundings as it is forming.
- Differences in the growth process affect the snow crystal differently. The crystal’s final form depends on factors such as how water molecules diffuse through the air surrounding a growing crystal and how water molecules adhere to the surface of the crystal.

- Japanese physicist Ukichiro Nakaya documented the influence of temperature and humidity on snow crystals by growing them in his lab in the 1930’s. He is credited with creating the first artificial snowflakes. Nakaya once said “Snow crystals are the hieroglyphs sent from the sky.”
- The Nakaya diagram shows that the largest, most photogenic stellar snow crystals only grow at temperatures of approximately −15° C (5° F).
- Needles and columns are best found at about −6° C (21° F).
- Capped columns appear when the temperature changes as the crystals grow.
- The more elaborate, branched crystals grow when the humidity is high.
- Simple prisms grow when the humidity is low or when the crystals are tiny.

- We don’t yet fully understand why snow crystals grow this way, but scientists are making progress.
- Researchers have discovered that the growth behavior of ice depends on the molecular structure and dynamics at the crystal surface. Scientists are still learning about this.