
Credit: IvanStoter, CC BY-SA 4.0, via Wikimedia Commons
Lithium-ion batteries have transformed our lives. They make our smartphones possible. They power our laptops and most other rechargeable devices—including, increasingly, our vehicles.
We’re even hoping to put giant batteries on the grid, to store solar- and wind-generated electricity when it’s being produced to use later when it’s not.
With that increased demand, lithium has become expensive. Its supply is volatile and controlled by just a few countries.
So, battery scientists are looking for an alternative. And they may have found it—in sodium. It’s a light metal related to lithium and is globally abundant and cheap, just 2% the cost of lithium.
And it can be used in similar ways—with advantages and disadvantages.
Sodium-ion batteries maintain most of their performance in cold temperatures, where lithium suffers.
They’re less prone to runaway thermal reactions, meaning fewer fires. And may be easier to recycle.
However, their energy density is lower than lithium and their lifespan is shorter, while their size and weight are greater.
This may make sodium batteries best suited for the grid. But scientists are working to improve them and hybridize sodium with lithium to leverage the strengths of both.
Combined designs could be smaller, longer lasting, safer, hardier and cheaper. Only time will tell if they could scale up enough to transform our electrical systems … again.
Background
Synopsis: Human civilization depends on battery technology for energy storage, but ever-present lithium-ion batteries require minerals that are often toxic, are geographically concentrated in countries where common mining practices may violate human rights, and are becoming increasingly expensive. Lithium’s chemical cousin sodium provides a potential alternative. Sodium-ion batteries have complementary characteristics but can be made from more-accessible minerals that are less toxic and cheaper to produce. However, their lower energy density and faster electrode degradation make them less than ideal for some applications.
- Batteries convert chemical energy into electrical energy using two electrodes (the cathode, marked “+,” and the anode, marked “-”) immersed in an electrolyte solution that allows the movement of ions but prevents direct contact of the electrodes.
- When a battery is recharged, the operation is reversed, and electrical energy is converted back into chemical energy.

A lithium-ion, 18650-size rechargeable battery (right) with an ordinary AA battery (left). The 18650 is most commonly found in laptop battery packs and in Tesla automobiles. One Tesla Model S battery pack uses 7,104 of these battery cells.
Credit: Lead holder, via Wikimedia Commons - Batteries can be made from a variety of materials. The most common types include nickel-cadmium (Ni-Cd), sealed lead-acid (Pb), nickel–metal hydride (NiMH), and lithium ion (Li-Ion).
- Solar and wind farms need to be able to store energy in industrial-scale batteries while the sun shines and the wind blows for energy production later when they can’t generate power.
- When a battery is recharged, the operation is reversed, and electrical energy is converted back into chemical energy.
- Lithium-ion batteries include lithium (Li), cobalt (Co), and nickel (Ni) for the cathode and carbon (graphite, C) for the anode.

A typical lithium-ion battery from a laptop computer.
Credit: Kristoferb, via Wikimedia Commons- Lithium is a critical component of these batteries as the charge carrier and is sourced principally from brines in Argentina, Chile and Bolivia (ED-008 Lithium Power).
- Cobalt is a toxic and cancer-causing byproduct of copper and nickel mining, with 70% of the world’s production coming from the Democratic Republic of Congo (DRC), where human rights abuses are a concern. Russia, Australia and Canada also have cobalt reserves.
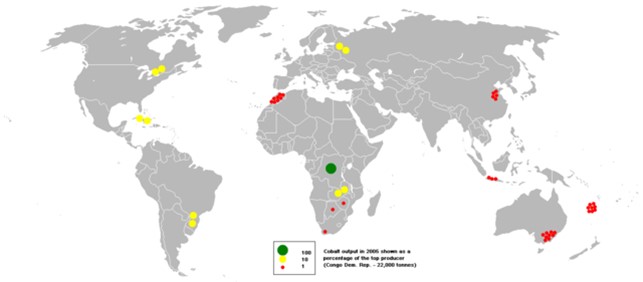
This bubble map shows the global distribution of mined output of cobalt in 2005 as a percentage of the top producer, the DRC.
Credit: Anwar saadat at English Wikipedia, via Wikimedia Commons - Nickel comes mainly from mines in Indonesia, Russia and the Philippines.
- Graphite provides a stable material for lithium-ion storage during charging and discharging, with 70% to 80% of the world’s supply coming from China.
- Sodium-ion batteries use sodium (Na), manganese (Mn), phosphorous (P), and carbonaceous material (C) for the anode.
- Sodium is an alkali metal like lithium, and while it has an extra orbital full of electrons, it acts like lithium in chemical reactions because of the single electron in its outer shell. In these batteries, sodium acts as the charge carrier instead of lithium. Sodium costs just 1% to 3% of what lithium costs and is globally abundant.
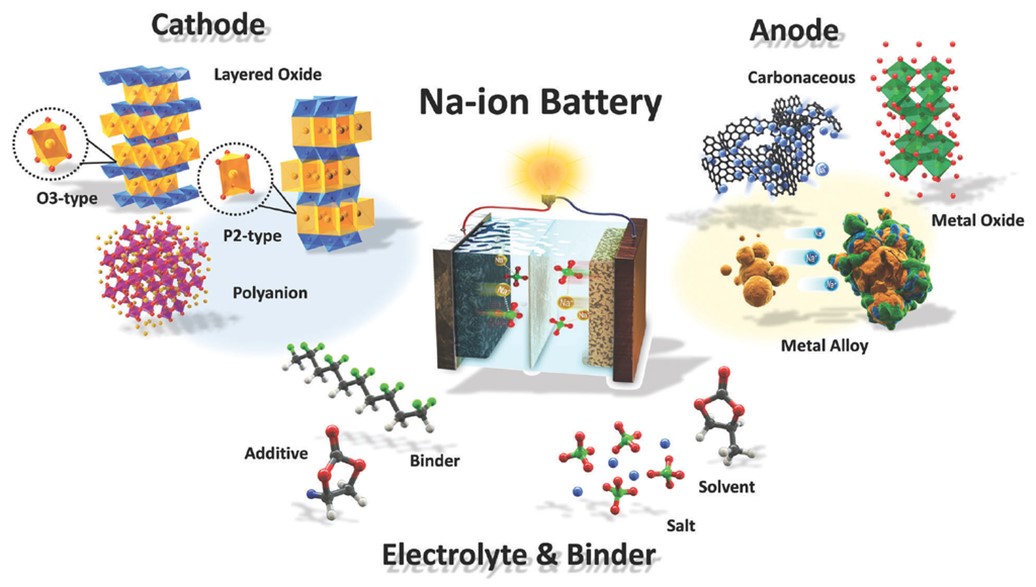
Illustration of a sodium (Na)-ion battery system with sodium species depicted in yellow.
Credit: Jang-Yeon Hwang, Seung-Taek Myung, and Yang-Kook Sun, via Wikimedia Commons - Manganese is mined in Gabon, South Africa, China, Mexico, Brazil, and Ukraine.
- Phosphorous is mined in North Africa, the Middle East, China, and Florida.
- Carbon-based materials are used in the anode to store and release sodium ions, and even organic carbon materials can be used in these batteries.
- Sodium is an alkali metal like lithium, and while it has an extra orbital full of electrons, it acts like lithium in chemical reactions because of the single electron in its outer shell. In these batteries, sodium acts as the charge carrier instead of lithium. Sodium costs just 1% to 3% of what lithium costs and is globally abundant.
- A comparison of sodium-ion batteries (Na-ion batteries) and lithium-ion batteries (Li-ion batteries) reveals strengths and weaknesses of each type of energy storage system.
- Lithium-ion batteries generally have higher energy density compared to sodium-ion batteries, meaning they can store more energy per unit of weight or volume, allowing for longer-lasting and more compact battery systems. However, recent breakthroughs are closing this gap. With a larger footprint required for the same energy delivery, the best use of sodium batteries may be the electric grid, where expansion is possible adjacent to a solar or wind farm.
- Sodium-ion batteries are considered safer than lithium-ion batteries because sodium-ion chemistry is less prone to runaway thermal reactions, which can lead to battery fires or explosions.
- Sodium batteries generally have faster electrode degradation.
- Lithium-ion batteries previously had a longer cycle life compared to sodium-ion batteries, but that may be changing. Cycle life refers to the number of charge–discharge cycles a battery can undergo while maintaining its performance.
- Sodium batteries maintain 90% of their performance at -4°F (-20°C), while lithium battery performance drops off considerably in freezing temperatures.
- The components of sodium-ion batteries can be recycled through a simple recovery process, while lithium-ion battery recycling may require complex separation of metals.
- Lithium is less abundant and geographically concentrated compared to sodium. This can result in potential supply chain challenges for lithium-ion batteries, especially as demand increases. Sodium is more readily available, reducing concerns over material availability and geopolitical issues, making sodium-ion batteries cheaper to produce once industrialized.
- Sodium-ion battery technology is cheap, safe, and ready to go.
- When commercialized, these batteries will be less expensive to produce because of their constituent minerals.
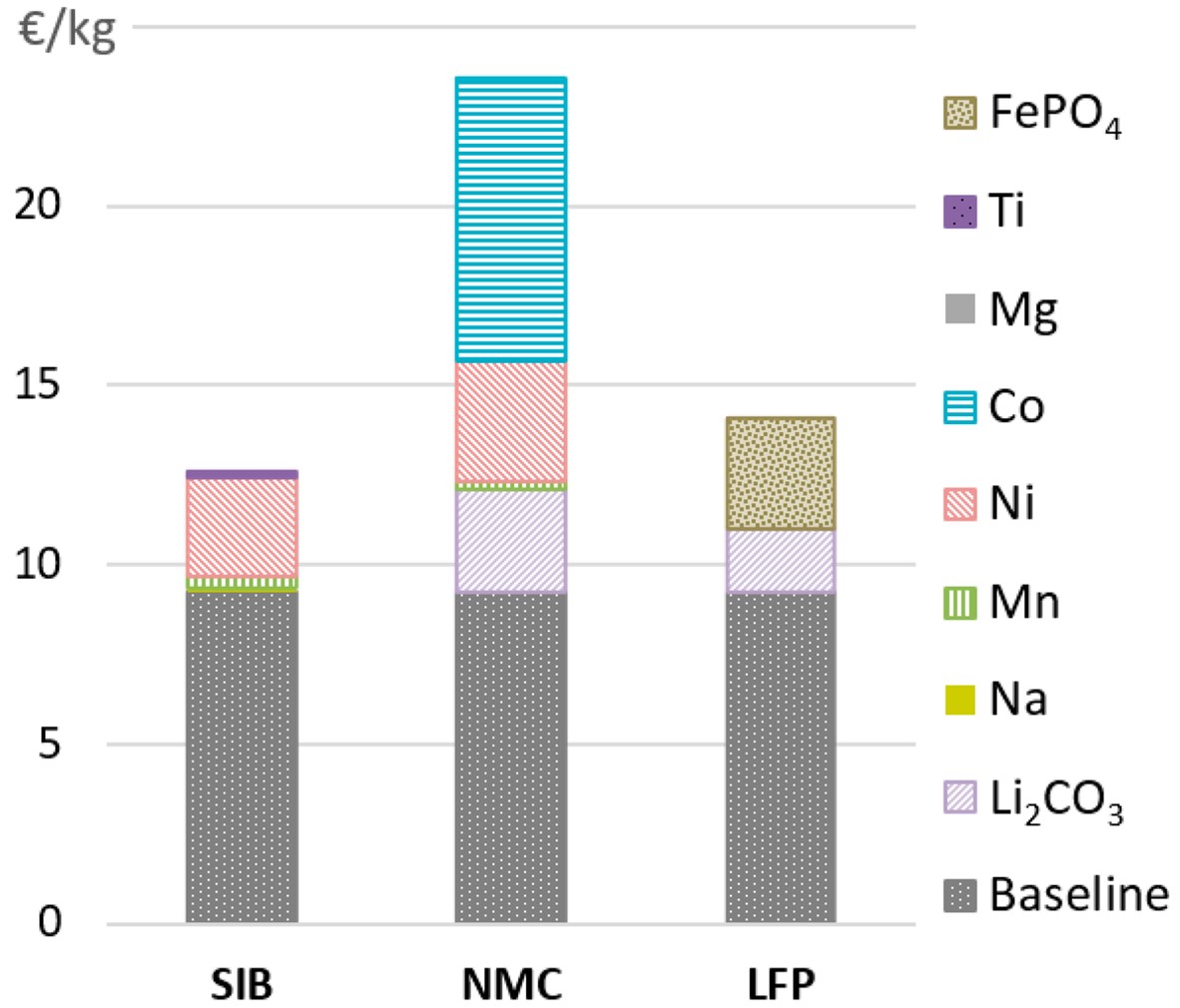
2019 cost comparison of sodium-ion batteries (SIB) and two types of lithium-ion batteries (NMC and LFP), broken down into components. Note the very high costs of cobalt and lithium.
Credit: Jens F. Peters, Alexandra Peña Cruz, and Marcel Weil, CC BY 4.0, via Wikimedia Commons - Chinese laboratories are currently at the forefront of industrializing the production of sodium-ion batteries, having worked out how to make sodium batteries with components so similar to lithium batteries that they can use the same manufacturing equipment.
- Chinese companies are also on the verge of commercializing mixed sodium-lithium battery systems to combine the weather resistance and low cost of sodium with the extended range of lithium.
- When commercialized, these batteries will be less expensive to produce because of their constituent minerals.

