
One hundred sixty years ago, scientists invented the spectroscope, which breaks light into its spectrum of colors.
They soon discovered that some elements, when heated, produce a signature light color. Hydrogen, for instance, makes orange.
When they pointed the spectroscope at the sun, they saw lots of orange—and some yellow, which didn’t match any element on Earth.
They named this alien element helium, after the Greek sun god Helios.
For decades, many doubted its existence. Until a scientist aimed the spectroscope at lava and saw the yellow again. Helium had been found on Earth.
Further study revealed that helium was being produced by the decay of uranium and trapped underground in reservoirs. It also revealed that it’s a very special element.
Helium is so light that Earth’s gravity can’t hold it. When released at the surface, it rises through the atmosphere into space.
It’s also inert: helium won’t bond with other elements, meaning it’s nontoxic and nonflammable. That makes it useful to create sterile, nonoxidizing environments for medical procedures, clean rooms, and welding.
Helium also enters its liquid state colder than any other element. Liquid helium is therefore used to cool superconductor magnets, and in MRI machines and nuclear colliders.
So next time you see helium balloons at a party, or inhale it to sing happy birthday in a chipmunk voice, remember that helium itself is an element worth celebrating.
Background
Synopsis: Helium is most familiar to us because of the fun it brings—from birthday balloons to squeaky voices—but it is essential for many medical and superconductor applications. In 1868, scientists found it in the spectrum of the sun, but it had never been seen on Earth. For more than a decade, scientists believed it was an alien element, until it was finally discovered in volcanic emissions from Mount Vesuvius in 1882. Helium is a finite resource so light that it escapes Earth’s gravity unless it is contained.
- About 150 years ago, while studying the spectrum of the sun, French astrophysicist Jules Janssen and English astronomer Norman Lockyer found a new element never before seen on Earth.
- In the mid-1800’s, scientists developed a new instrument called a spectroscope that was similar to a telescope but worked like an ultra-high-powered prism to disperse incoming light into its component wavelengths.
- Scientists found that every element produces a unique spectrum when heated that works like a fingerprint to confirm the presence of specific chemicals.
- Because scientists knew the emission spectra of elements found on Earth, like hydrogen, they could figure out the composition of light sources like stars.
- When observing the spectrum of our sun, they could see the presence of hydrogen as an orange line, but in 1868 they found a yellow line that had never emerged from elements on Earth—a new alien element! Scientists called it helium after the Greek name for our sun, Helios.
- For decades, many questioned helium’s existence, doubting that an element could exist in space but not on Earth.
- Finally, in 1882, an Italian physicist named Luigi Palmieri found the same yellow line while analyzing the spectrum of lava flows from Italy’s Mount Vesuvius.
- Scottish chemist William Ramsay later showed that earthly helium gas is a byproduct of the radioactive decay of uranium, which occurs principally in granitoid rocks.
- Helium is a limited resource on Earth because it is lighter than air—so light that Earth’s gravitational field is not strong enough to hold it.
- If it makes its way to Earth’s surface outside of a container, it escapes into space, ultimately swept away by the solar wind.
- Helium is used in lifting technologies. If it is collected in a relatively light container, like a birthday balloon or dirigible, it can lift its container skyward.
- Helium is inert; its outer orbital is completely full with its two electrons, so it can’t react with other elements.
- Because it is inert, helium is nonflammable. The Hindenburg Zeppelin disaster of 1937 is thought to have resulted from using highly flammable hydrogen to lift the massive aircraft. Today’s blimps use helium.
- Although it is lighter than helium and often used in weather balloons, hydrogen’s extreme reactivity—with just one electron in its orbital—makes it easily trapped on Earth’s surface by being incorporated into molecules ranging from water to complex carbon-rich compounds to hydrated rock-forming minerals.
- Helium is used in welding to provide an inert atmosphere that protects metals from oxidation and other reactions that might occur rapidly at high temperatures.
- Because it is inert, helium is nontoxic, so it can be used in medical applications—and to amuse your friends.
- Inhaling the nontoxic helium changes your voice into a squeaky quack. Swapping out the oxygen- and nitrogen-rich air in your vocal tract for helium, with its higher sonic velocity, amplifies the higher-frequency components of your voice. But be careful not to do this repetitively. If you replace the air in your lungs with helium too often, you can deprive yourself of oxygen and pass out.
- Helium has many unique properties with applications in advanced technologies
- Helium is so small that it is used to flush systems and locate microscopic leaks.
- It is used to purge rocket-propulsion systems of liquid oxygen and hydrogen fuels because it is inert and can sweep out very small spaces.
- Helium will escape through even the smallest leak, so it is used to test high vacuum and fuel systems.
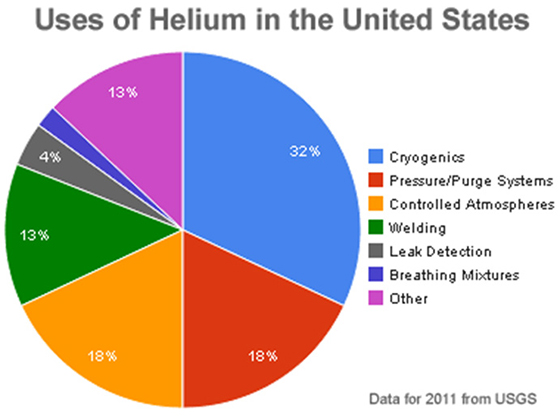
- Helium is used to create a controlled inert atmosphere for manufacturing perfect crystals and optical fiber.
- Most importantly, helium is essential to superconductor technologies in its liquid form because it is inert and because no other element can exist as a liquid at temperatures so close to absolute zero, or 0o Kelvin (-273.15oC or -459.67oF).
- At 0.95 oK (-272.2oC or -457.96oF), helium’s melting point is the lowest of all elements. Its boiling point is 4.25oK (-268.9oC or -452.02 oF).
- Superconductors conduct electricity with little to no resistance, enabling the generation of very large magnetic fields.
- Liquid helium is necessary to cool the magnets used in many modern technologies, including MRI machines and CERN’s Large Hadron Collider on the border of France and Switzerland.
- Most importantly, helium is essential to superconductor technologies in its liquid form because it is inert and because no other element can exist as a liquid at temperatures so close to absolute zero, or 0o Kelvin (-273.15oC or -459.67oF).
- Helium is renewed very slowly over geologic time; for practical purposes, it is a finite nonrenewable resource.
- Helium evolves over millions of years as certain radioactive elements common in granitic rocks decay during the process of fission (atom splitting).
- For commercial volumes, helium can only be trapped where granites fractured enough to allow the gas to migrate upward occur below extremely impermeable caprocks, like salt or anhydrite—which can trap the tiny helium molecules before they can escape to Earth’s surface and beyond.
- The United States currently produces about 75 percent of the world’s helium supply. Other commercial accumulations of helium occur in Qatar, Algeria, Australia, Russia, Tanzania, Poland, China, and Canada.
- Improving superconducting technologies so they can operate at more easily accessible temperatures will help to conserve Earth’s limited helium supply.
- Even though it is rare on Earth, helium makes up 25 percent of the atoms in our universe.
- On the sun, helium forms from fusion of hydrogen atoms; the sun’s huge gravitational field contains both gases.
- Helium accounts for 8.9 percent of the total atom count for our sun, while hydrogen makes up 91 percent, leaving less than 0.1 percent for all other elements.

