
Credit: http://birdphotos.com, CC BY 3.0, via Wikimedia Commons
Background
Synopsis: Sulfur has a reputation for being the stinky troublemaker behind rotten eggs and volcanic fumes, but there’s much more to this element than meets the nose. From the proteins in your body to the fertilizers that grow your food, sulfur is everywhere—whether you notice it or not!
- Sulfur compounds are known for producing some of the stinkiest smells to humans.
- Hydrogen sulfide, H2S, is a colorless gas with an overpowering stench that reeks of rotting eggs. It seeps naturally from hot springs, volcanoes, and swamps and is the byproduct of decomposing plant matter. This noxious gas is also responsible for the stomach-turning odors of manure pits and sewers.
- Sulfur in fossil fuels like oil and coal creates another offensive-smelling gas when burned: sulfur dioxide (SO2). This pungent, throat-burning gas – often compared to the acrid smell of burned matches – escapes from industrial smelting of metals like copper, zinc, and lead, as well as from volcanic eruptions and hot springs.
- Organosulfur compounds, a vast group numbering in the millions, are a major source of nature’s worst odors. These carbon-based sulfur compounds invade our noses in unexpected ways.
- Thiols, a particularly vile group of organosulfur compounds, are the culprits behind some of the most nauseating natural odors. They are responsible for the skunk’s infamous defensive spray, and the sharp, eye-watering scent of freshly chopped onions.
- Allyl methyl sulfide, another organosulfur compound, linger in the breath long after garlic has been eaten, giving rise to the dreaded garlic breath that no mint can fully mask.
- Even Benjamin Franklin took note of sulfur’s impact on the senses when he observed “a few stems of asparagus eaten shall give our urine a disagreeable odor.” Today, we know that organosulfur compounds are to blame for this pungent transformation - though not everyone’s nose is sensitive enough to detect it.

If you have visited Yellowstone National Park, you undoubtably recall the smell of hydrogen sulfide gas produced by thermal features like Mammoth Hot Springs.
Credit: Brocken Inaglory, CC BY-SA 3.0, via Wikimedia Commons
- Not all sulfur has this unpleasant smell. Pure elemental sulfur is a bright yellow, odorless, tasteless, brittle solid and is insoluble in water.
- Sulfur, from Latin meaning “burning stone,” is the tenth most abundant element on Earth and its combustibility has made it useful for thousands of years.
- In Earth’s crust, sulfur is the fifth most abundant element, found in many ores such as gypsum (CaSO4• 2H2O), pyrite (Fe2S), cinnabar (HgS), and galena (PbS), and sphalerite (ZnS)!
- The ancient Greeks used burned sulfur, also known as brimstone, as a fumigant, a pesticide that would kill insects, rodents, weeds and other pests.
- The volcanic Mount Etna in Sicily provided an ample supply of the mineral which was then burned to generate sulfur dioxide. This form of sulfur was then used as a preservative for wine and for bleaching cloth.
- The Bible mentions brimstone fifteen times from Genesis through Revelations and is cited as a punishment for the wicked of “fire and brimstone.”

Sulfur crystals from the El Desierto mine in Bolivia.
Credit: Ivar Leidus, CC BY-SA 4.0, via Wikimedia Commons
- Today, we obtain sulfur from either the Frasch process or from the refining of oil and natural gas.
- The Frasch process is a method for extracting high-purity sulfur from underground deposits.
- Superheated steam (170°C or 338°F) is injected underground at 10 atmospheres of pressure, melting the crystalline sulfur.
- Compressed air pushes the molten sulfur to the surface through a separate pipe.
- This process yields sulfur that is over 99% pure.
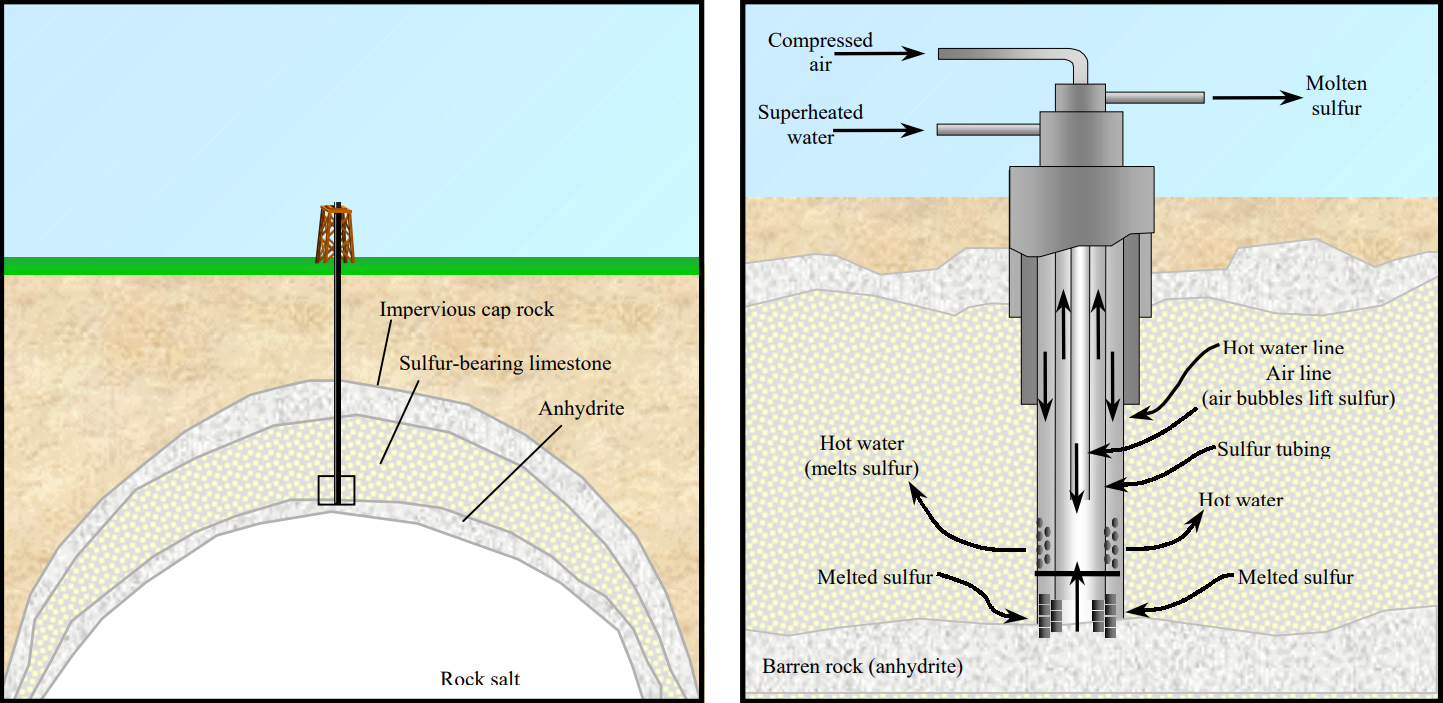
The Frasch process uses steam and pressure to extract sulfur from underground deposits.
Credit: Joyce A. Ober, public domain, via Wikimedia Commons
- Sulfur is an impurity in the crude oil and natural gas that must be removed to prevent environmental and industrial issues.
- If left in fuel, burning sulfur-rich diesel and fuel oil releases sulfur oxides (SOx) which:
- React with water to form acid rain, harming ecosystems and decreasing photosynthesis rates in plants.
- Irritate the respiratory system, worsening conditions like asthma.
- Corrode refinery equipment and pipelines, increasing maintenance costs.
- To prevent these issues, sulfur is removed using processes like:
- Hydrotreatment (uses hydrogen to break sulfur bonds)
- Adsorption (traps sulfur on solid surfaces)
- Oxidations (converts sulfur into a removeable compound)
- Originally developed to comply with environmental regulations, these sulfur-removal processes now make oil and gas refining the world’s primary source of sulfur.
- Ironically, sulfur compounds known as thiols or mercaptan are added to odorless methane and propane. Adding the rotten egg smell helps to detect leaks and prevent accidents.
- If left in fuel, burning sulfur-rich diesel and fuel oil releases sulfur oxides (SOx) which:
- Sulfur is a necessary nutrient for all living organisms, including humans. It is a key component of two essential amino acids, cysteine and methionine, which are required for building proteins in the body.
- Cysteine helps produce collagen, which affects skin elasticity and texture. It is also a major component of beta-keratin, the protein found in hair, skin, and nails.
- Methionine plays a vital role in metabolism and antioxidant activity, helping protect cells from damage.
- A diet rich in meat, fish, eggs, nuts, and beans provides the necessary sulfur-containing amino acids to support overall health.
- In addition to nitrogen, phosphorus, and potassium, plants require sulfur for essential biological processes.
- Sulfur plays a key role in chlorophyll synthesis and nitrogen fixation, which are critical for plant growth.
- Legume crops, such as beans and peas, require high amounts of sulfur to produce protein efficiently.
- Certain aromatic crops, such as cabbage and onions, need extra sulfur to develop their distinctive smells and flavors.
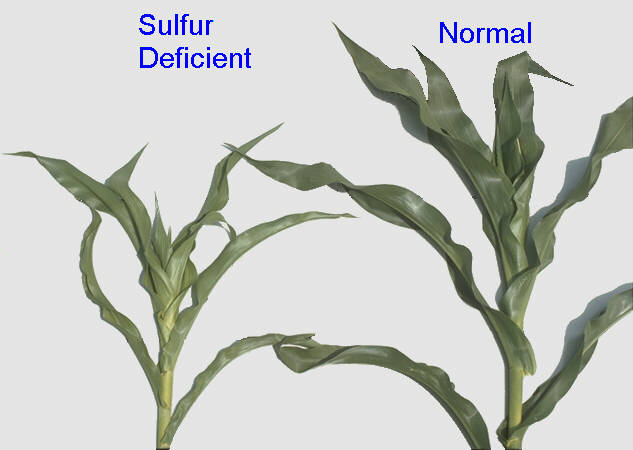
A deficiency of sulfur causes yellowing and less growth in corn and other plants. Ironically, with the reduction of sulfur from industry due to increased environmental protections, farmers are now needing to amend fields with added sulfur.
Credit: Phillip Barak via University of Wisconsin
- Beyond its biological necessity, sulfur is a crucial element in a vast range of industrial applications.
- The very smelly mercaptans are added to odorless natural gas so that even the smallest leak could be detected and responded to.
- Fertilizer Production: Sulfuric acid is the most-produced chemical in the world, primarily used in manufacturing inorganic phosphate fertilizers to enhance soil fertility.
- Metal Processing: Sulfuric acid is essential in refining iron, copper, and steel. It is used in steel pickling, a process that removes rust and carbon impurities from metal.
- Battery Production: Sulfuric acid, combined with lead, generates electricity in car batteries and large industrial batteries.
- Chemical Manufacturing: Sulfuric acid is a precursor to many other chemicals, including hydrochloric acid, nitric acid, synthetic detergents, pigments, and dyes.
- Explosives and Fireworks: Sulfur is a key ingredient in gunpowder, matches, and fireworks.
- Rubber Vulcanization: Natural rubber is soft and sticky. When heated with sulfur, it undergoes vulcanization, which makes it harder, more durable, and elastic.
- Agriculture: Sulfur is widely used as a fungicide and insecticide to protect crops from pests and diseases.
- Medicine and Health:
- Sulfur is used as a disinfectant and in antibiotics that treat bacterial and fungal infections.
- Sulfur-based ointments help treat acne, rosacea, eczema, dandruff, warts, skin discoloration, and shingles.
Sulfur may be best recognized for its stink, but it’s also essential for life! From building proteins in our bodies to helping plants grow and fueling major industries, this element does it all.
Episode script
Cow manure. Garlic breath. Asparagus pee. What do they have in common?
They all stink. And that stink comes from sulfur.
Hydrogen sulfide is a very pungent gas that forms when plant matter decomposes, and gives its telltale fragrance to sewage, rotting garbage, and swamps.
Sulfur dioxide – the smell of burned matches – is a gas produced when fossil fuels burn, metals are smelted, volcanoes erupt or hot springs bubble up.
Organosulfur compounds -- there are millions in nature -- create some of its most repellent aromas. Like a skunk’s spray or the eye-watering fumes of freshly chopped onions.
The ancient Greeks burned sulfur as a fumigating pesticide to kill insects and rodents. When combined with water, sulfur fumes become acid rain.
But sulfur is not all bad news. In fact, it’s essential to life on Earth.
Sulfur is a key component of two essential amino acids, cysteine and methionine, which are building blocks for proteins.
Cysteine helps produce collagen and beta-keratin, proteins found in skin, hair and nails. Methionine protects cells from damage.
Plants too require sulfur, to make chlorophyll and fix nitrogen, which is why it’s used as fertilizer.
Legumes use sulfur to make their protein. Cruciferous plants, like cabbage and broccoli, get from sulfur their signature flavor – and of course, their stinky aroma.

