
Credit: Felix Mittermeier, via Wikimedia Commons
Phosphorus is one of the five elements essential for life. It’s represented on the periodic table by the letter P.
Phosphorus is key to building bones and teeth and as the building block for the fuel that powers our growth and movement. You might remember adenosine triphosphate, or ATP, from high school biology class.
Each day the human body makes, uses and recycles its entire weight in ATP to drive our cellular function.
Phosphorus is not very common on the surface of Earth, where it’s mostly found in phosphates, which are compounds of phosphorus and oxygen.
These are not very reactive—or biologically useful. So, living things have to get their phosphorus from something else.
Scientists are now studying more reactive, useful compounds of phosphorus called phosphides. Many of these occur in meteorites. And as we’ve talked about on other episodes, in Earth’s distant past, the planet was pummeled by them.
Scientists now think that much of the biologically available phosphorus on Earth came from these ancient meteorites.
Phosphates can also be converted into bioavailable phosphides when they’re struck by lightning.
Early Earth may have had 10 times the lightning as today, which could also have unlocked phosphates in rock and soil for use by lifeforms.
P may be for phosphorus. But thanks to meteorites and lighting, P is also for life.
Background
Synopsis: Phosphorus (chemical symbol P) is necessary for life. It is in your DNA and RNA and is a vital ingredient of the compound that enables energy transfer among cells, allowing for growth and movement. It is part of cell walls and is especially important for vertebrate bones and teeth. Despite its importance in biology, it is not as common as might be expected on Earth’s surface. Scientists have long wondered about the availability of phosphorus for the origination of Earth’s early life-forms because phosphates are fairly unreactive. Recent studies suggest that more-bioavailable compounds called phosphides may have been “heaven-sent” in meteorites or flashed into existence by early Earth’s intense lightning storms.
- Phosphorus is one of the five elements essential for life, along with hydrogen, oxygen, carbon and nitrogen.
- Phosphorous is element 15 in the periodic table—look for the P.
- It is not found as a free element on Earth because it is highly reactive.
- Phosphorous glows a faint white as it reacts when exposed to oxygen, so it was named phosphorus, Greek for “light-bearer.”
- Phosphorescence describes the tendency of a substance to glow after illumination.
Credit: NEUROtiker, via Wikimedia Commons
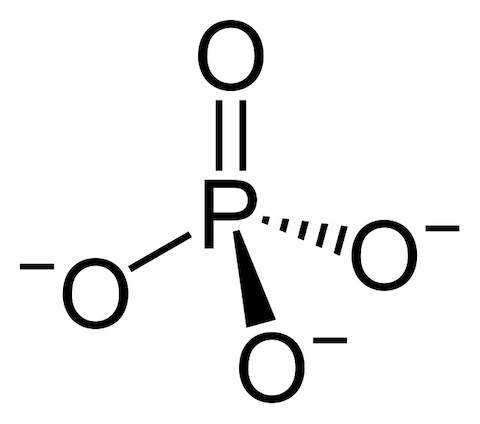
- On Earth’s surface, phosphorus usually occurs as phosphate (PO4).
- Phosphate is strongly oxidized and is the lowest energy state for phosphorus in an oxygen-rich environment.
- It is unique, with three negatively charged oxygen atoms that easily form bonds.
- Phosphate is a critical part of the sugar–phosphate backbone of both DNA and RNA, connecting the genetic bases into their structural long chains.
- It is integral to the phospholipids that form cell walls and is a key constituent of the mineral apatite that builds vertebrate bones and teeth.
- Phosphate is at the core of the fundamental fuel of life that powers both growth and movement, adenosine triphosphate (ATP). ATP provides the energy that drives many cellular processes, from chemical reactions to nerve impulses and muscle contraction. The human body makes and uses its weight in ATP every day.
- All Earthly organisms must find a source of phosphorus.
- Animals get their phosphorus from eating plants (or other animals that eat plants), and plants get their phosphorus from soil.
- Soil is a mixture of decaying organic matter and sediments that come from the erosion of local rocks.
- For gardeners, fertilizers are described by the proportions of their three most important chemical constituents: nitrogen (N) to phosphorus (P) to potassium (K), or N-P-K. For example, a fertilizer with balanced constituents would be described as a 10-10-10 fertilizer.
- Supplementing soils with phosphorus encourages blooming and fruit production while increasing root development and feeding key microbes that unlock nutrients from the soil for the plants.
- Humans get phosphorus from foods like beans, seeds, nuts, eggs, seafood, poultry and dairy.
Credit: Bonniemf, incorporating work by NASA Earth Science Enterprise, via Wikimedia Commons
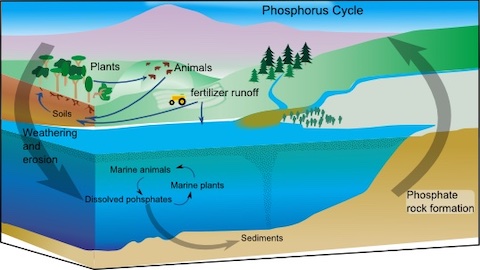
- The phosphorus cycle is one of the slowest of Earth’s biogeochemical cycles, operating in geological time.
- Phosphorus excreted by animals or not used by plants runs off into lakes, rivers and the ocean.
- It is used in the life cycles of marine organisms and is ultimately locked away in marine sediments, where it is lithified into rocks.
- Millions of years later, tectonic forces may return these marine rocks to Earth’s surface, where weathering makes their phosphorus accessible to life once again.
- The U.S. Geological Survey (USGS) reports that 85% of known inorganic phosphate reserves are in Morocco, with other accumulations in China, Russia, Scandinavia, French Guiana and the U.S. states of Tennessee, Florida Idaho and Utah.
- Because phosphorus is available in excrement and decaying organic matter, sources such as compost, urine, manure, bone ash, bat guano and even coprolites (fossilized dung) historically have been used as fertilizer.
- As humans move fertilizer, food and related effluent from farms to cities around the globe, we have changed the natural phosphorus cycle.
- Phosphorus is one of the five most-abundant elements in biological systems and is thought to be the limiting nutrient for the capacity of Earth’s biosphere.
- However, it is less common in the universe and is surprisingly scarce in Earth’s oceans. Compared to hydrogen abundance, for every phosphorus atom in …
- … the cosmos, there are 2.8 million hydrogen atoms, making phosphorous the 17th-most-abundant element in the solar system.
- … the ocean, there are 49 million hydrogen atoms.
- … bacteria, however, there are only 203 hydrogen atoms, demonstrating that the element is concentrated in the biosphere.
- However, it is less common in the universe and is surprisingly scarce in Earth’s oceans. Compared to hydrogen abundance, for every phosphorus atom in …
- On Earth’s surface, the calcium phosphate mineral apatite [Ca5(PO4)3(F,Cl,OH)] is the principal source of phosphorus, but it is not very reactive.
- However, the apatite minerals (hydroxyapatite, fluorapatite and chlorapatite) are fairly inert and insoluble in water.
- Hydroxyapatite is the principal component of our sturdy bones and teeth.
- If phosphate is so unreactive, how did phosphorus become so essential for life?
- In a 1959 essay, Isaac Asimov called phosphorus “life’s bottleneck.”
- Scientists are studying other less-oxidized compounds of phosphorus that are more reactive, including metal-rich phosphides.
- Phosphides form in places where metals are abundant and oxygen is scarce.
- They are not very common on Earth’s surface, but Earth’s core contains abundant phosphides.

Credit: University of South Florida via NASA
- Iron-nickel phosphides, including schreibersite [(Fe, Ni)3P], occur in meteorites. Early Earth was pummeled by these types of space rocks.
- When schreibersite is exposed to water, researchers have found that phosphorus–oxygen bonds may develop, making the phosphorus bioavailable to life.
- Over time, phosphides become oxidized into phosphates. Up to 10% of phosphate on Earth’s surface is thought to have originated from meteorites.
- Phosphides also form when phosphate-rich soil or sediments are struck by lightning, turning unreactive phosphates into bioavailable phosphides.
- Lightning strikes create special rocks called fulgurites (from fulgur, Latin for lightning) that may range from a few feet to more than 60 feet long.
- Today we see about 500 million lightning flashes per year, and about a quarter of those strike the ground. Scientists estimate that 4 million years ago, early Earth may have seen 1–5 billion lightning flashes per year.
- Phosphorus may have only been made available for biological processes because meteorites and lightning flashed through Earth’s early atmosphere to unlock it.

