
Credit: NIST
How long is a second? That depends on how you measure it.
In a previous EarthDate, we talked about how people divided the day into 24 hours, then hours and minutes into 60. That made the second 1/86,400th of a day.
But how long is a day? Turns out, that’s not constant. Earth wobbles slightly on its axis, changing its rotation time.
So scientists settled on a year, reasoning the day’s length would average out.
But which year? Those aren’t constant either.
So they agreed on a specific year: 1900.
A second then, was measured as 1/31,556,925.9747th of the year 1900. Not a very elegant solution.
So scientists looked for a new measurement.
They found an isotope that changes magnetic orientation when, and only when, it’s hit with microwave energy of a very specific frequency.
This relationship was so stable, and the procedure so repeatable, that they used it to set the new standard of time: 1 second equals 9,192,631,770 cycles of the microwave energy that switches the atomic state of cesium-133.
That may not be any more elegant. But it is very precise—down to the nanosecond.
Background
Synopsis: What is a second? Time can be measured from the coarse scale or the fine scale. The coarse-scale approach starts with the length of a day and divides it up into 86,400 seconds. The fine-scale approach starts with a very consistent measurement of the frequency required to cause energy level transitions in certain atoms. For cesium-133, this frequency is more than 9 trillion cycles per second. How do we measure the second today?
- Dynamic time uses the motion of planets and stars to track time.
- It ties time to the rotation of a variety of celestial bodies and to the movement of stars crossing our night sky.
- A simple, age-old, yet elegant method of defining the second uses dynamic time tied to Earth’s rotation.
- Historically, Earth’s daily rotation was divided into 24 hours. Each hour was divided into 60 minutes, and each minute was divided into 60 seconds.
- Doing the math: 24 hours × 60 minutes × 60 seconds = 86,400 seconds in a day, so each dynamic second has to be 1/86,400th of a day long.
- If only it was that easy.
- Tidal friction from the moon puts the brakes on Earth, slowing the time it takes our planet to make a complete rotation by about 18 millionths of a second each year.
- As we learned in a previous EarthDate, the length of Earth days has varied over time. About one billion years ago, a day was just 20 of our modern hours. 200 million years from now, a day may be about 25 of our hours.
- The length of the day may also exhibit shorter-term variations from other factors: from cyclical variation of Earth’s axial tilt and wobble, to uneven weight distribution of snow and water in different hemispheres.
- The second is the base unit we use to define time, so it must have a constant duration.
- Metrologists, scientists who study time measurement and standards, knew the long-term variation of the dynamic second was not acceptable for modern timekeeping.
- In 1960, scientists proposed tying the length of the second to the length of the year, reasoning that the duration of Earth’s orbit around the sun is more stable than the length of the day.
- They had to designate a specific year, so they selected the exact length of the year that began at the start of January 1900, defining what was called the ephemeris second as 1⁄31,556,925.9747th of that year.
- However, producing a reliable global reference to a past astronomical event was not easy, so ephemeris time was only the standard for about 7 years.
Credit: National Physical Laboratory
- To improve the stability of timekeeping, in 1967, scientists turned away from the macro-scale of the solar system and toward a precise, consistent natural phenomenon at the nanoscale. They defined the cesium second in what is known as atomic time, or SI (International System of Units) time.
- Scientists figured out that the key to defining a constant second was to identify a precise natural frequency that can be detected and measured consistently and independently, attaining the same results even with different equipment and in different locations.
- Frequency is defined as cycles per second, so once a specified frequency is attained, it can be converted into seconds by counting the number of cycles.
- Atomic time produces a constant second by finding the precise frequency that forces certain atoms to change energy states.
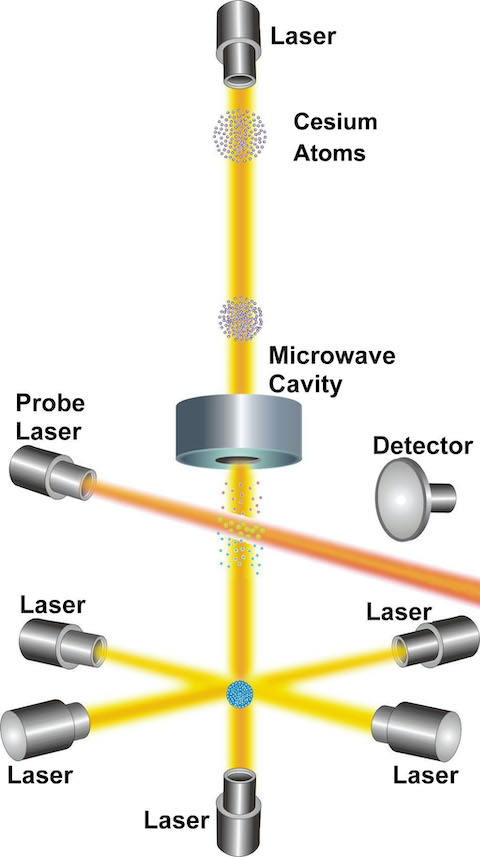
Credit: NIST
- Cesium-133 was chosen for use in early atomic clocks because, as an alkali metal, it has a single electron in its outer orbital, making it easy to “interrogate” with an external frequency that can force it to change atomic energy states. Additionally, it has an energy transition frequency that is high enough to provide precision measurement of a second.
- The single electron in the outer shell of a cesium-133 atom will align magnetically either in the same direction as the spin of the nucleus or opposite to the spin of the nucleus.
- Jumping between these alignments is called a hyperfine energy transition, and this jump can be triggered in a cesium-133 atom when it is perturbed or “interrogated” by microwave energy with a very specific frequency of 9,192,631,770 cycles per second, or Hertz (Hz).
- Scientists can easily detect whether the atoms have changed energy states by interrogating them a second time with an optical frequency from a laser. If the atoms emit a photon, it’s evidence that they changed states.
- Once atomic clock operators “dial into” the right frequency and count 9,192,631,770 cycles, that’s considered one second, no matter where you are on Earth. However, there are adjustments that must be made for gravitational variation at different locations on land and in space.
- The exact 1967 definition of a second from the International Committee of Weights and Measures says: "The second is the duration of 9,192,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the cesium-133 atom."
- Setting this cesium second as the actual length of a dynamic second in 1967 provided a seamless transition from dynamic time to atomic time for the public.
- Some sources describe the frequency phenomenon behind atomic clocks as a natural atomic-scale “pendulum,” but this is not the case.
- Atoms do not emit a frequency, so there is no frequency to be measured.
- Scientists measure the externally generated frequency that causes the atoms to change their energy state.
- Since 1967, the atomic second has remained the same length. However, that didn’t solve all the challenges of keeping our atomic clocks aligned with Earth’s actual seasons.
- Whenever both Earth’s varying rotation and orbit and atomic time are about to become out of sync by 0.5 seconds, metrologists arrange for a “leap second” to be added at midnight on the last day of December or June. This keeps the two systems in sync.
- The first leap second was added on June 30, 1972, and the most recent adjustment was on December 31, 2016.
- The next leap second may be added in the next few years, but because of the variability of Earth’s rotation, we can’t say when for certain. Those determinations are made by the Earth Rotation and Reference Systems Service (IERS) based on the actual difference they observe about 6 months before each upcoming correction date.
- The NIST-F1 atomic clock in Boulder, Colorado, is a cesium fountain clock, which slows the movement of the atoms by cooling a cesium-133 gas cloud to near absolute zero (0 K). This enables even more precise microwave interrogation to cause the atomic transitions, resulting in a more stable clock.
- Early cesium-133 clocks were so stable, they would have lost only 1 second every 300,000 years. But by 1999, atomic clocks like the NIST-F1 were so precise, they would only lose 1 second every 20 million years.
- The NIST-F2 is an even newer model, which has its chamber encased in a liquid nitrogen chamber. Both NIST-F1 and NIST-F2 are located at the National Institute of Standards and Technology in Boulder, Colorado.
- These two particle physics primary standards in the United States are averaged together with others around the world to provide the reference for timekeeping mechanisms used in Coordinated Universal Time (UTC).
- UTC is the internationally agreed upon time that is used in the automatic setting of your smartphone time, computer clock, radio-controlled clocks in your home, and the satellites that enable global navigation and communication.
Credit: NIST
- Over the past two decades, scientists have continued to improve atomic time accuracy to boost computational calibration, enabling practical results like GPS systems tuned to navigation precision on a centimeter scale.
- Optical atomic clocks use high-frequency laser light to cause atomic transitions. Since the frequency of light waves is 10,000 times greater than that of microwave radiation, optical measurements result in much greater precision.
- Optical lattice clocks use atoms of elements with higher transition frequencies than cesium, like strontium and ytterbium, that are trapped in a lattice of laser beams to increase consistency and precision.
- Ion clocks use single aluminum and mercury ions and are similarly precise—more than 100 times more accurate than the clocks that currently keep UTC standard time.
- Today, our most accurate clock uses a single positively charged aluminum ion cooled to nearly absolute zero and trapped within electromagnetic fields.
- The ion absorbs ultraviolet light at a frequency of more than a quadrillion beats per second, enabling measurement of very small slices of time.
- This clock achieves an amazing level of stability: In over 33 billion years, it would only be off by 1 second.
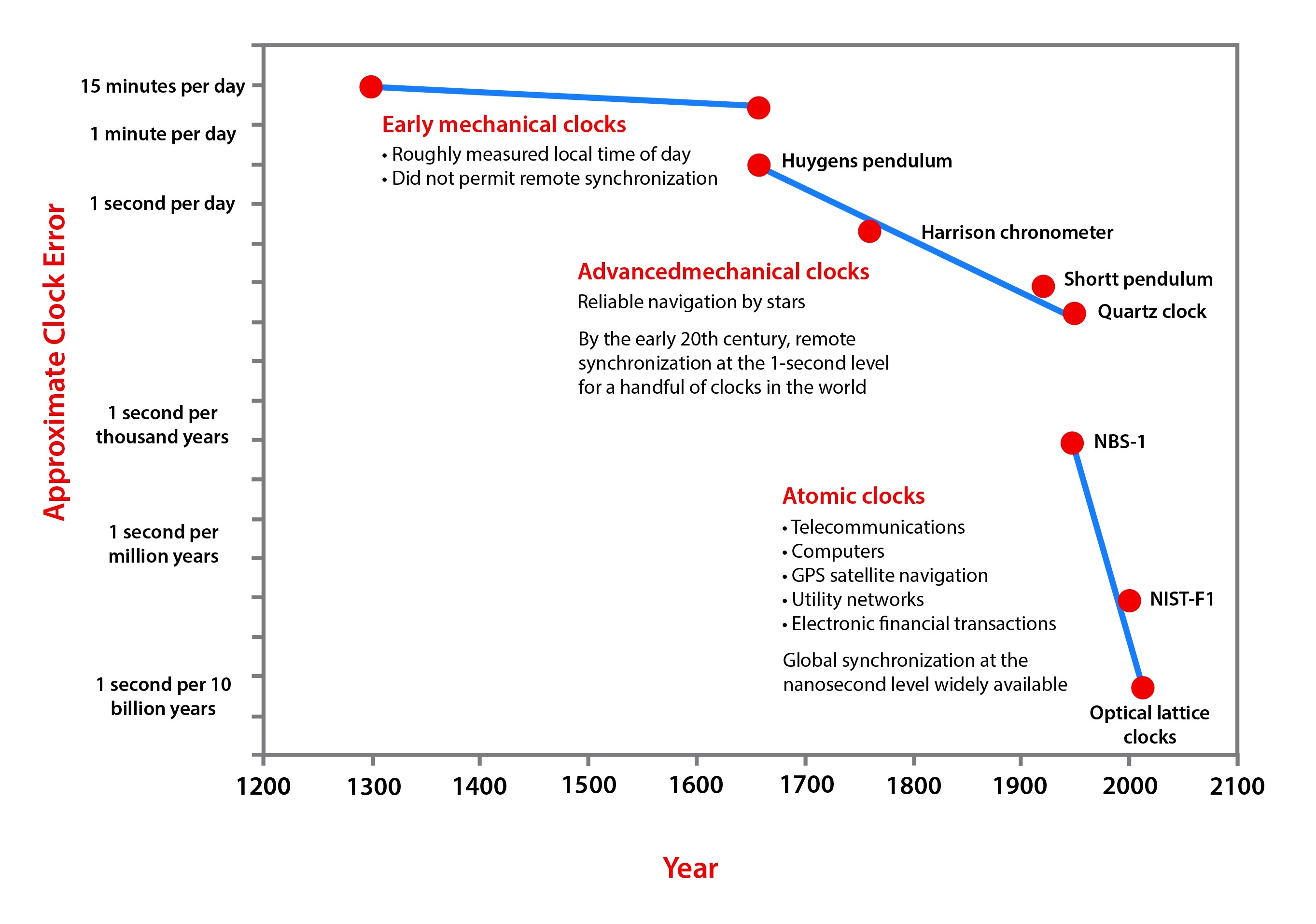
Credit: NIST
- Development of ultra-accurate optical clocks is very rapid, and metrologists expect that they will be used to redefine the second over the next decade.
- With ongoing innovation in the field, it is not certain what type of optical clock will become the new standard for the future.
- Ion clocks are less influenced by environmental changes but are sensitive to quantum “noise.”
- The thousands of atoms in lattice clocks are more susceptible to the environment but average out the influence of quantum noise.
- These clocks are so precise that they are being used to test Einstein’s theories of relativity and to search for dark matter.
- With ongoing innovation in the field, it is not certain what type of optical clock will become the new standard for the future.

