
Credit: Bureau of Land Management, public domain, via Wikimedia Commons
For billions of years, there was no fire on Earth.
There was heat to ignite it, like volcanic eruptions and lighting strikes. But fire lacked two very crucial ingredients: fuel to burn, and oxygen to feed the flames.
Ironically, plants would provide both.
Fire is a chemical reaction where fuel oxidizes to produce heat and light energy, carbon dioxide, water vapor and other gases.
Fire can start when there’s about 13% oxygen in the air, but it won’t stay lit.
It will burn steadily at 16% oxygen, improving to about 23%, and plateauing at 30%.
Land plants evolved 470 million years ago, consuming carbon dioxide to perform photosynthesis and producing oxygen as a by-product.
As they grew, evolved, and covered more of the Earth, plants finally drove Earth’s atmosphere to above 13% oxygen.
And, 420 million years ago, the first fires started.
Plants continued to thrive, and swampy forests eventually covered the globe during the time of dinosaurs. Oxygen levels hit 29% and wildfires became very common.
Around 50 million years ago, oxygen levels stabilized at 21%, as fire and photosynthesis struck a balance: Fire consumes plants and oxygen and makes carbon dioxide. Plants consume carbon dioxide and make oxygen.
A perfect cycle.
Background
Synopsis: Fire is a chemical reaction that requires an ignition source, fuel and oxygen, producing heat, light, ash and smoke. For billions of years, there was no fire on Earth—even with an effective ignition source like lightning, fire could not happen until there was enough fuel and oxygen to sustain the reaction. But once land plants evolved to use photosynthesis to turn carbon dioxide into oxygen, wildfire became possible, turning that oxygen back into carbon dioxide. These two chemical reactions have balanced the level of oxygen in Earth’s atmosphere for more than 300 million years, sustaining terrestrial life—like us.
- Fire is a chemical reaction, possibly the most important to life on Earth after photosynthesis.
- Wildfire is the combustion of plant-matter fuel in the presence of oxygen.
- The history of wildfire on Earth is intimately linked to the history of photosynthesis on Earth.
- Fire is combustion, which is an exothermic oxidation reaction. This means that oxygen combines with fuel, releasing heat and light energy along with combustion products like smoke and ash.
- Before anything can happen, the reactants must reach ignition temperature—putting fuel next to oxygen will not cause a fire without ignition.
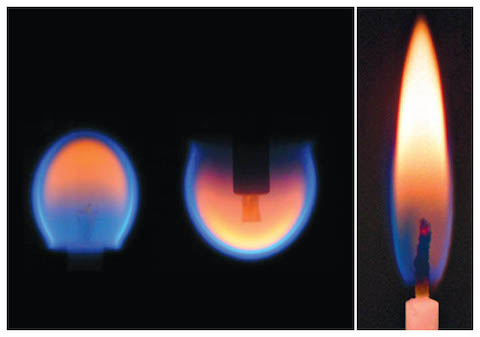
- Wood has to reach about 300°F (150°C) before it begins to evolve its volatile gases in smoke, leaving behind charcoal. When you burn charcoal in a barbecue grill, it does not smoke because charcoal is what is left after heating evolves all the volatile gases from wood but has not yet consumed the wood.
- Once the volatile gases are hot enough at about 500°F (260°C), combustion occurs: The carbon in the gas combines with oxygen from the air to produce carbon monoxide (CO), and if there is enough oxygen, the reaction also produces carbon dioxide (CO2). Hydrogen from the fuel also combines with oxygen to produce water (H2O).
- The exothermic reaction evolves heat energy, and light energy is produced by the incandescent property of carbon atoms, with hotter flames appearing as blue and cooler flames appearing as orange or yellow.
- The carbon in charcoal combines with oxygen, burning slowly and maintaining ignition temperature to keep the fire going until all the fuel is spent, leaving nonflammable residue behind as ash.
Credit: NASA
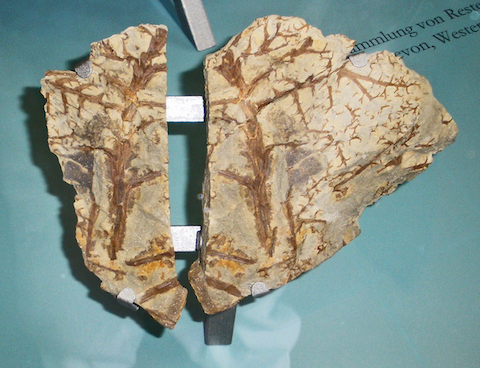
- Evidence of ancient wildfire comes from fossils of charcoal, known as fusain, which is a silky carbon residue left behind when charcoal is buried and fossilized. Although fusain may be interlayered with coal, it is not coal.
- Coal is a plant-rich rock that forms when ancient swamps are buried and lithified. As it is buried deeper and deeper, it progresses from peat to lignite to bituminous coal to shiny black anthracite. All of these produce soot when burned.
- Fusain may occur along with coal in ancient swamps that occasionally burned before they were buried.

Credit: Paul Stocksdale, CC BY-SA 3.0, via Wikimedia Commons
- For billions of years, fire could not occur on Earth because the reaction requires three essential ingredients that took time to evolve: ignition sources, oxygen and fuel.
- Fire requires an ignition source, a way to get the reactants up to ignition temperature.
- Natural ignition sources have been operating for most of Earth history, including lightning strikes, volcanic activity and volcano-induced lightning, meteor impacts and rockfalls that generate sparks.
- Fire requires enough oxygen to combine with, or oxidize, its fuel. Earth’s early atmosphere did not have enough oxygen to support fire until about 400 million years ago.
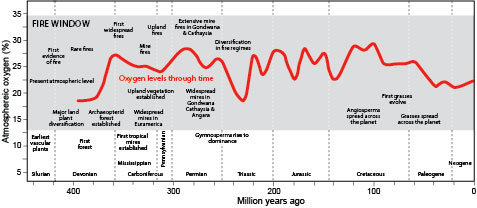
- Researchers have found that without at least 16% atmospheric oxygen, fires may be ignited but will not stay lit. Burn probabilities remain low through 19%, ramping up to 23%, then plateauing at 30%.
- Today’s atmospheric oxygen level is 20.9%.
- Fire requires fuel, and natural wildfire exploits cellulose-rich plant matter for fuel. Ironically, it was plants that built up oxygen levels in the atmosphere to the point that fire could consume them.
- The first tiny land plants evolved 470 million years ago in the Middle Ordovician Epoch, consuming carbon dioxide during photosynthesis and producing oxygen as a waste product, initiating the accumulation of oxygen in Earth’s atmosphere.
- It took 50 million years before atmospheric oxygen reached 13%, just barely enough to sustain limited fire. Late Silurian fossils composed of leafless, scrubby plant charcoal from 420 million years ago are the oldest evidence of fire on Earth.
- About 400 million years ago, in the Middle Devonian Epoch, Earth “greened up” as large forests evolved to take over the landscape at the same time that atmospheric oxygen finally exceeded 16%.
- Fire requires an ignition source, a way to get the reactants up to ignition temperature.
Credit: Ghedoghedo, CC BY-SA 3.0, via Wikimedia Commons
- With higher oxygen levels and plenty of plant matter, the stage was set for more-frequent large-scale wildfires. Wildfire frequency can be tied to atmospheric oxygen abundance.
- In the oxygen-rich Carboniferous Period, famous for its huge, swampy, coal-forming forests in the Northern Hemisphere, 10–20% of the coal by volume is fossil charcoal, indicating frequent, widespread fires.
- Then, in the Triassic Period, atmospheric oxygen dropped below 20%, correlating with a dramatic reduction in the amount of fossil charcoal, suggesting there were fewer fires.
- In the late Triassic, Jurassic and Cretaceous, evidence of fire became significant again as atmospheric oxygen exceeded 26%, this time turning flower-bearing angiosperms into charcoal.
- In the Cenozoic Era, oxygen levels declined to around 21% during the Eocene Epoch and have been fairly steady since then, for 55 million years.
- Six to seven million years ago, grasslands expanded, providing more tinder for fires that sustained those grasslands, producing a warmer climate conducive to drying fuel, leading to further fire development. These fires would have been the first that hominids may have experienced. We’ll talk about that in another EarthDate.
- Today, 40% of Earth’s surface is covered with wildfire-prone ecosystems.
Credit: K. Cantner, AGI.
- Beyond shaping Earth’s ecosystems and climate, fire may be essential to stabilize atmospheric oxygen levels within a range that sustains terrestrial life (such as humans).
- Photosynthesis consumes carbon dioxide, producing oxygen as a waste product.
- As oxygen increases in the atmosphere, wildfires increase in their frequency, severity and extent, releasing carbon dioxide and reducing the biomass capable of photosynthesis.
- If emerging plant growth can access enough of the right nutrients, the cycle begins again.

