Herbivores eat plants, and carnivores eat animals that eat plants, to get their energy.
Plants and many bacteria create their own energy through photosynthesis, which depends on chlorophyll…which requires nitrogen.
As we’ve described in other episodes, most nitrogen on Earth is in the form of N2, the inert nitrogen gas that won’t bond with other compounds, making it useless to plants.
So, most plants rely on bacteria that cling to their roots and split the nitrogen in the soil for them. But when they can’t get enough, humans help them out, with nitrogen-rich fertilizers.
Early fertilizers relied on naturally concentrated nitrogen. For instance, bat colonies eat insects, which contain nitrogen, then excrete nitrogen-rich droppings. For centuries, humans harvested tons of bat guano to fertilize our crops.
But demand outstripped supply, so we had to invent a new source. At the start of the twentieth century, two German chemists devised a way to combine the nitrogen in air with hydrogen produced from natural gas to form ammonia, NH3. And their work won a Nobel prize…
Because liquid ammonia could then be used to mass produce nitrogen-rich fertilizers. This drove the massive expansion of industrial agriculture—which has both created and allowed us to feed a growing global population.
Background
Synopsis: Whether a vegan or a paleo dieter, all humans depend on photosynthesizers that form the base of our food web. That makes us dependent on chlorophyll—the green pigment that is essential for photosynthesis—and chlorophyll needs nitrogen. Nitrogen is abundant in the air we breathe, but it exists as molecules of paired nitrogen atoms that can’t react to form compounds until they split apart. Lightning, bacteria and fungi make that happen naturally, while humans create reactive nitrogen for fertilizers. Nitrogen greens the earth but too much can harm ecosystems.
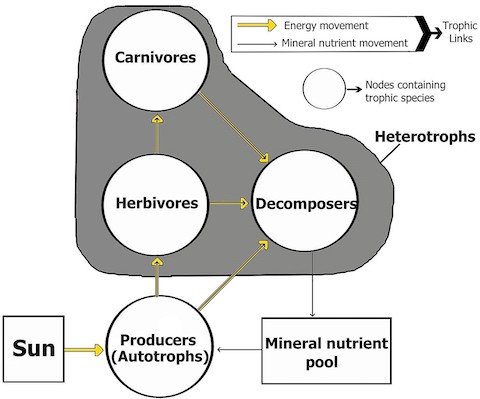
- Terrestrial food webs rely on primary producers of biomass, like plants, to support consumers and decomposers that are unable to create their own biomass from sunlight.
- Plants, algae and many bacteria are autotrophs, producing organic compounds from carbon dioxide and light energy through photosynthesis.
- Plant-eating herbivores, meat-eating carnivores, and decomposers like fungi are heterotrophs that must consume biomass originally created by primary producers for energy.
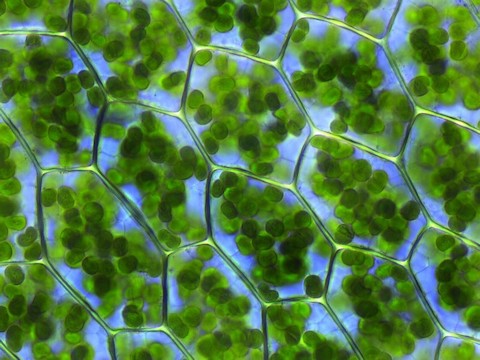
- Photosynthesis uses the energy from sunlight to turn carbon dioxide and water into carbohydrates, the food source for plants.
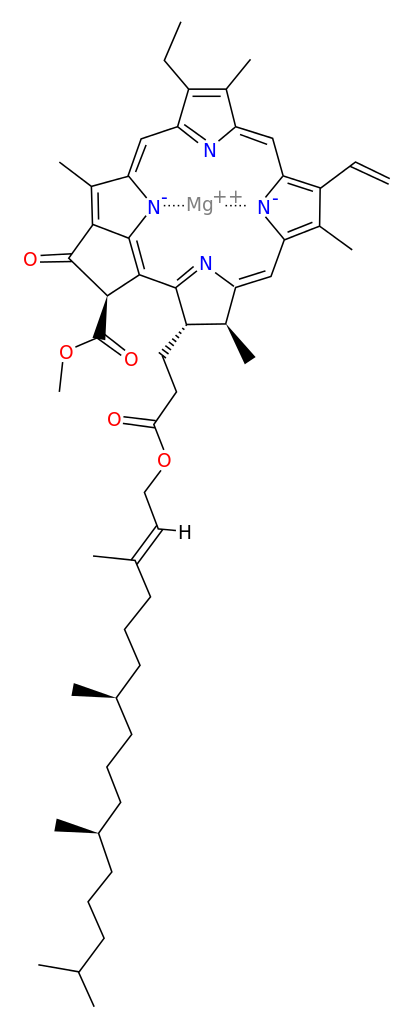
- Photosynthesis requires chlorophyll molecules that produce the characteristic green color of plants.
- Nitrogen and magnesium are required for a key component of chlorophyll that turns sunlight into the energy that drives photosynthesis.
- Nitrogen nourishes plants, greening leaves and producing new cells that increase stem and leaf size and accelerate growth, so it is an important ingredient in fertilizer.
- While nitrogen is the most abundant gas in our atmosphere, it is not directly available to organisms.
- Nitrogen has an atomic number of 7 with an atomic weight of 14.0067.
- Earth’s crust only has about 19 ppm of nitrogen, on par with lithium, but rock weathering is an important source of nitrogen.
- Earth’s atmosphere contains 78% nitrogen, followed by oxygen at 21%, argon at 0.9% and other gases at 0.1%.
- However, atmospheric nitrogen occurs as N2, an inert gas that can’t form compounds.
- By contrast, atmospheric oxygen (O2) is a gas that is so reactive that it’s highly flammable, as described in a prior EarthDate episode (ED-228).
- The N2 (dinitrogen) molecule is bonded by three strong covalent bonds (N≡N), meaning two nitrogen atoms share three electrons in their outer shells.
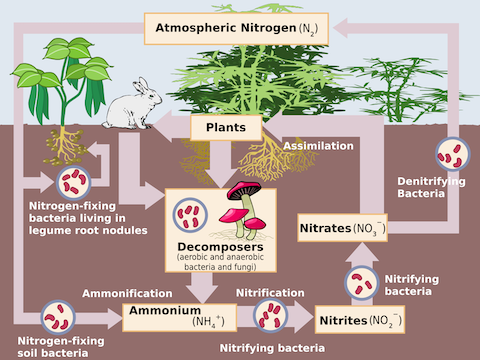
- So, before it can make useful compounds, atmospheric nitrogen must be split apart, or fixed, to form more reactive nitrogen compounds that are able to participate in chemical reactions.
- Nitrogen is found in amino acids that form proteins as well as in nucleic acids like DNA.
- Nature has a few amazing mechanisms that produce reactive nitrogen.
- In a previous EarthDate episode (ED-027) we talked about how lightning disassociates atmospheric dinitrogen to create nitrogen oxides that dissolve and are delivered to plants in rainwater.
- Nitrifying bacteria in soil are the key link that plants depend upon for nitrogen. The bacteria cling to plant roots, absorb nitrogen from air and convert it to soluble nitrogen bearing compounds that plants can use.
- Animals and decomposers consume plant matter and produce ammonia in waste products, making more nitrogen available.
- Fertilizers accelerate plant growth and mitigate one of the most common global agricultural problems—chlorosis—a nitrogen deficiency that yellows plant leaves.
- Early fertilizers were made from natural sources, like bat guano and saltpeter, and demand quickly outpaced supply as farmers sought to feed growing nations.
- In 1909, Fritz Haber figured out how to produce ammonia (NH3) from atmospheric nitrogen and hydrogen using a metallic catalyst under high pressure and temperature conditions.
- In the 1910’s, Carl Bosch helped Haber to upscale the process to industrial levels using osmium as the catalyst.
- The Nobel prize winning Haber-Bosch process solved key challenges for large-scale continuous-flow high pressure technology, making ammonia available to be liquified into agricultural fertilizers, as well as wartime explosives.
- About 176 million tons of ammonia are produced every year using the Haber-Bosch process.
- Nitrogen puts the green in Earth, benefiting plants and animals, but too much green can create environmental imbalances, some with major consequences for life.
- Too much nitrogen may cause plants not to produce flowers or fruit (for instance, lawn fertilizer may prevent nearby bushes from flowering), and their leaves may turn yellow and drop off.
- Nitrogen-enriched wastewater leached from agricultural fields may drive extreme bacterial growth in water bodies—a severe type of pollution called eutrophication—depleting oxygen so that the water column is deadly to higher organisms.
- To minimize impact, using organic waste or slow-release fertilizers instead of traditional industrial products may slow the release of nitrogen into aqueous systems.
- Introduction of ammonia oxidizing microbes could reduce nitrogen compounds but must be carefully balanced to avoid overuse that starves crops of the nitrogen they must have.

