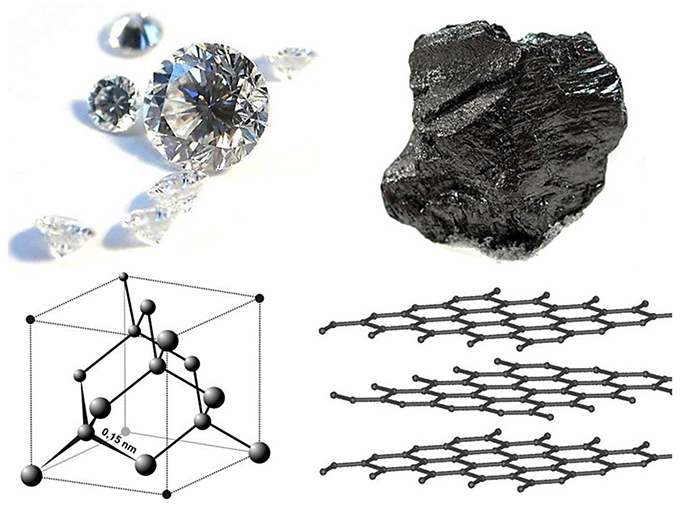
You’ve heard of carbon copies, but what about carbon opposites? Diamonds and graphite are both made of pure carbon, but like a hero and a villain played by the same actor in a B movie, they’re almost comically different.
Diamonds are transparent, prized for their clarity and brilliance. Graphite is opaque and nearly black, valued for its ability to leave marks—which is why it’s used as pencil lead.
Diamonds are famously hard. This makes them highly abrasive, and used on saw blades, drill bits, and sandpaper. Graphite is incredibly soft and is so nonabrasive that it’s commonly used as a lubricant.
Diamonds are an excellent heat conductor, while graphite can be used as an insulator.
How could two minerals from the same element be such different characters? The answers lie in where they were born and how they were formed.
Diamonds are created deep within Earth under intense heat and pressure. Their crystal structure is bonded tightly together in three dimensions. Graphite is formed from organic matter when it’s heated during continental plate collisions. Its mineral structure is in sheets, which are bonded loosely together and can slip past one another.
Different minerals made of the same element are called allotropes and are more common than you’d think. There are other natural and man-made allotropes of pure carbon. All have different characters depending on their crystal structure—and one of them will likely change our lives. We’ll talk more about it on a future EarthDate.
Background
Synopsis: The diamonds in your jewelry and the graphite in your pencil lead are both made of pure carbon—but their properties are nearly opposite. That’s what different arrangements of the exact same atoms will do. Carbon’s many allotropes are remarkable and impact our daily lives.
- Carbon is the fourth-most-abundant element (by mass) in the universe and the second-most-abundant in our bodies because it can form long chains with hydrogen ions—making it essential for life on Earth and the basis of the science of organic chemistry.
- But carbon has an even bigger secret in the inorganic world—it has extremely different properties depending on how the carbon atoms are arranged in the carbon crystals.
- When pure forms of the same element differ in their crystal structure, they are called allotropes.
- Diamond and graphite—pencil lead—are the two best-known natural allotropes of carbon, with surprisingly different properties.
- Diamonds are pure carbon, with atoms packed together densely by very strong atomic bonds; graphite’s carbon atoms are arranged in strongly bonded sheets with weaker bonds between them that allow them to slide past each other.
- As a result, diamonds are the hardest mineral, and graphite is one of the softest; diamond is the ultimate abrasive, while graphite is a great lubricant.
- Diamond is clear and transparent, with brilliant optical dispersion that we treasure in gem-quality stones; graphite is black and opaque, leaving layers behind as marks and smudges on paper when we write with it.
- Diamond is an excellent thermal conductor; some forms of graphite are used for thermal insulation in heat shields and firebreaks.
- Diamond is an excellent electrical insulator, with all four of its electrons bonded strongly in a three-dimensional form; graphite is the most electrically conductive of the nonmetals—commonly used as the anode in lithium-ion batteries.
- Most gem-grade diamonds formed deep in Earth’s mantle at extremely high pressure, while graphite forms when organic matter is heated and metamorphosed during the mountain building of subduction and continental collision.
- There are several other natural and man-made allotropes of pure carbon, all with distinctive attributes depending on their 3D or 2D arrangement in sheets, spheres, or tubes.
- Some of the newly engineered allotropes of carbon will change our lives—but more on that next time!

