Credit: YouTube channel Smarter Every Day 2
Here’s a fun experiment you can try at home to make miniature cold lightning using rocks from your yard, or candy.
First, look for some quartz pebbles. Preferably milky white ones.
Next, find some wintergreen Life Savers—or white sugar candies will do.
Then grab some safety glasses, because you’ll be smashing things.
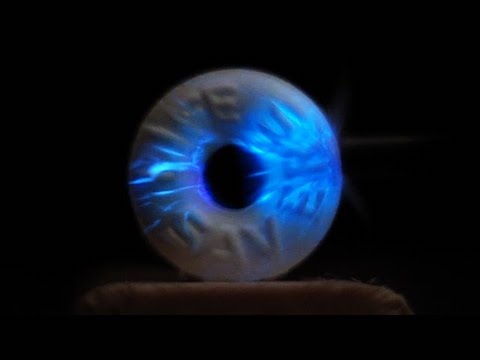
Now go to a darkened room, let your eyes adjust, and rub the pebbles together. Try different levels of friction and speed. You should see a flash of light in one or both pebbles as friction causes them to luminesce—without producing a spark.
This is called triboluminescence, which happens when some materials rub together or break.
Now try crushing the Life Savers with a hammer or pair of pliers. The wintergreen oil will also fluoresce, increasing the effect.
Scientists are still not exactly sure how this happens. It’s thought that the friction or cracking causes positive and negative charges to separate. When they recombine in the presence of nitrogen in the air, they produce cold light, without heat, similar to lightning in a cloud.
Besides being a neat trick, researchers are trying to put it to use. They’re experimenting with integrating triboluminescent materials into high-value items, like bridge structures or spacecraft parts.
Using special sensors that can see the faintest luminescence, they hope to observe fractures developing before critical failures could occur. A cool benefit from cold lightning.
Background
Synopsis: We usually think that light and heat go together, but “cold light” is generated in some situations that do not involve heat. Certain materials will light up when crushed, not because they are creating sparks, but because of microscopic-scale lightning that occurs as electrical charges separate then interact with nitrogen in air. You can get a good laugh out of demonstrating this effect with rocks, tape and even sugar candies. Materials scientists use it to provide warnings before potential catastrophic failures occur in high-value structures like bridges and spacecraft.
- Luminescence is the emission of light from a substance that has not been heated. It is sometimes called “cold light” and comes in various types.
- Bioluminescence is caused by biochemical activity in animals and insects such as fireflies. We will talk about that in a future EarthDate episode.
- Mechanoluminescence is caused by mechanical action on a solid. It includes triboluminescence, when frictional forces like scratching, crushing or rubbing cause materials to glow; fractoluminescence, when a substance is fractured; and piezoluminescence, when a material is deformed.
- The three types of mechanoluminescence, from friction, fracturing and deformation, were first observed 400 years ago but are still not well understood. They may be caused by the same mechanism.
- The effect was first noted around 1620 by Francis Bacon when he observed the effect in sugar crystals as they were broken.
- His findings were validated by Robert Boyle in 1663.
- The first documented historical use of the effect was by the Uncompahgre Utes of Colorado. Their ceremonial rattles were filled with clear quartz crystals that would flash through the buffalo rawhide when shaken in nighttime ceremonies.

Credit: Waya sahoni, public domain, Wikipedia
- Some scientists describe the effect as “very small–scale lightning”—a real tempest in your teapot!
- When certain crystalline materials are stressed, researchers postulate that positive and negative electrical charges are separated, creating electric fields.
- As the electrical charges recombine, they collide with nitrogen molecules, exciting electrons that release energy as a flash of light, similar to what happens in lightning-charged clouds (ED-027 Lightning Strikes).
- Like lightning, the light emitted is mainly in the invisible ultraviolet (UV) region of the electromagnetic spectrum, extending slightly into the visible deep-blue region.
- This luminescence only occurs in the presence of nitrogen—the effect will not occur in a vacuum.
- It is more easily observed when nearby substances fluoresce, absorbing UV light and re-emitting it in the visible range.

Credit: Dietrich Zawischa, Institute for Theoretical Physics Liebniz University Hannover
- There are several ways to demonstrate the effect. All of them benefit from observation in a darkened room once your eyes have adjusted for a few minutes.
- Find two quartz pebbles that you can hold in your hands and make sure they are dry. Milky pebbles that are nearly transparent produce the best triboluminescent effect.
- Head to a dark spot and let your eyes adjust.
- Tribo- comes from the Greek word for “to rub,” so rub one of the pebbles really hard across the surface of the other.
- Look for a flash deep within one or both of the pebbles.
- Try different speeds and pressures.
- If you have eye protection, scratch a pebble with a nail or bang them together to see different effects.
- Other minerals that will produce triboluminescence include calcite, diamond, feldspar, fluorite, micas, amblygonite and sphalerite.
- As Francis Bacon found, sugar is another crystal that emits light when crushed. Some candy will luminesce, especially wintergreen Life Savers; the methyl salicylate in wintergreen oil enhances the UV light by re-emitting it as blue-green fluorescence, which is an easier color to see in the dark.
- Buy a roll of Wint-O-Green Life Savers, grab your safety goggles, a mirror, pliers and a hammer and head to your darkened room.
- Once your eyes have adjusted, put your goggles on and crush a mint in the pliers, or smash one with a hammer.
- You can even put one in your mouth and watch in the mirror as you crunch it with your teeth. The effect is better if your mouth is drier: saliva may reduce the effect.
- This works with other hard sugar candies, but it works better with white ones than with clear ones. It will not work with sugar-free candy.
- Maple syrup sucrose produces especially strong luminescence.
- An additional material that exhibits triboluminescence is sticky tape.
- You will need a roll of sticky tape or duct tape and a darkened room.
- Tape is made up of a backing of fabric or plastic, a primer that helps the adhesive stick to the backing, the adhesive and a release coating on the opposite side of the tape to prevent the adhesive from sticking to the backing too strongly.
- Van der Waals forces cause tape to adhere, and when tape is peeled off a roll, charge separation occurs and electrons give off photons.
- In the presence of air, nitrogen molecules become excited at the location where the tape is detaching, where you will see a faint blue line of flashes. The effect is stronger if you put two sticky sides together then pull them apart.
- Researchers at UCLA have shown that without nitrogen around, as a roll of Scotch tape is unpeeled in a vacuum, no luminescence occurs. Instead, the tape emits enough x-rays to view the bones in a finger.
- Find two quartz pebbles that you can hold in your hands and make sure they are dry. Milky pebbles that are nearly transparent produce the best triboluminescent effect.

Credit: Carlos Camara and Juan Escobar
- Triboluminescence has not been studied as much as other material properties because there did not seem to be a good commercial application for it. But that is changing.
- Researchers are working on embedding triboluminescent ingredients into composite materials to help detect early stages of structural failure.
- Sensors detect triboluminescence generated where strain is causing microscopic deformation prior to catastrophic failure in high-risk situations like spacecraft, aircraft, ships, buildings, bridges and dams.

